Method for producing aromatic carboxylic acid by m-xylene and ethylbenzene multi-step co-oxidation
A technology for meta-xylene and aromatic carboxylic acids, applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate, etc., can solve problems such as difficult to generate phenylacetic acid, difficult to implement, and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0075] The present invention will be further described in detail below with reference to the accompanying drawings and embodiments. It should be noted that the following embodiments are intended to facilitate the understanding of the present invention, but do not limit it in any way.
[0076] 1. Production of m-xylene
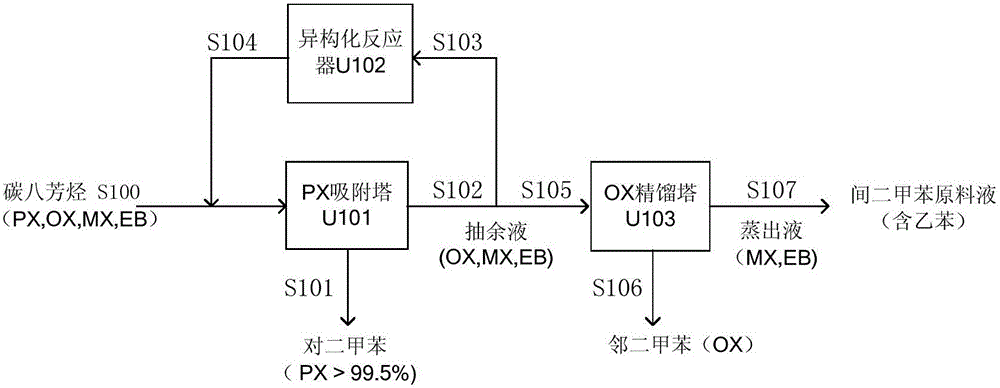
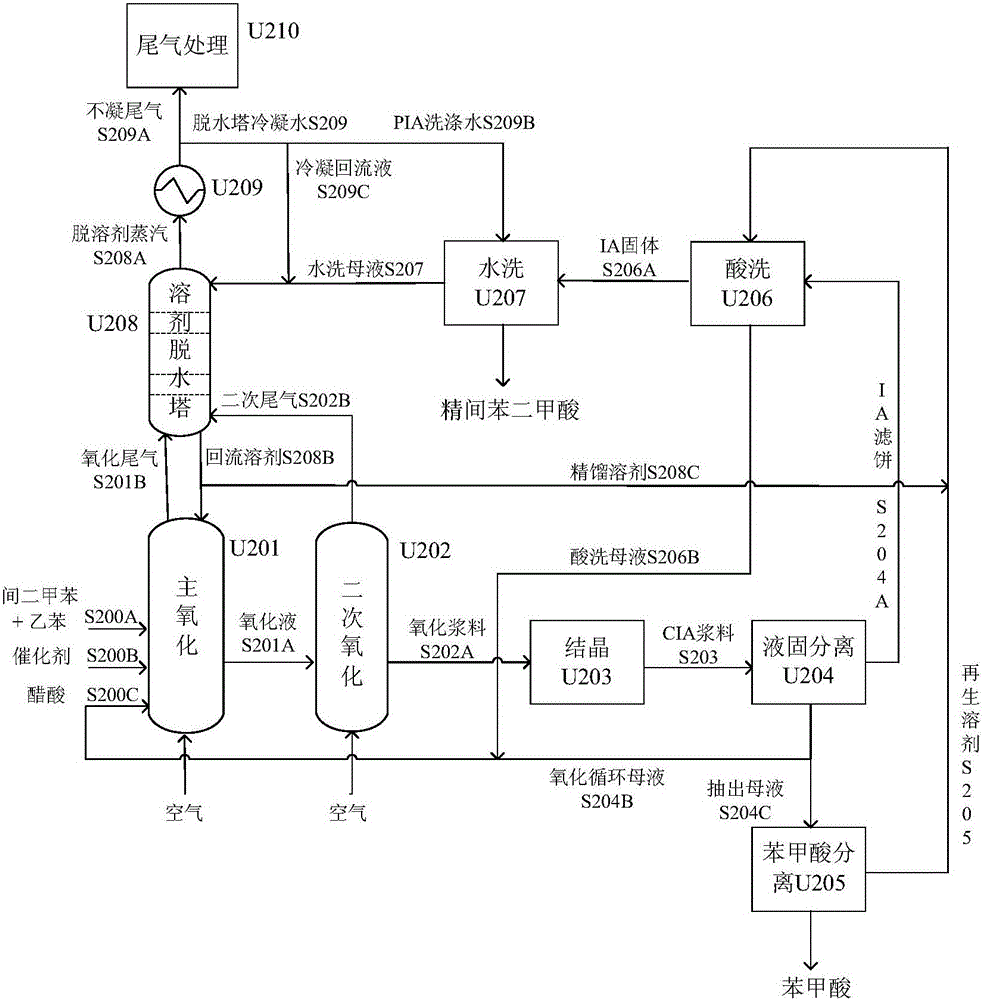
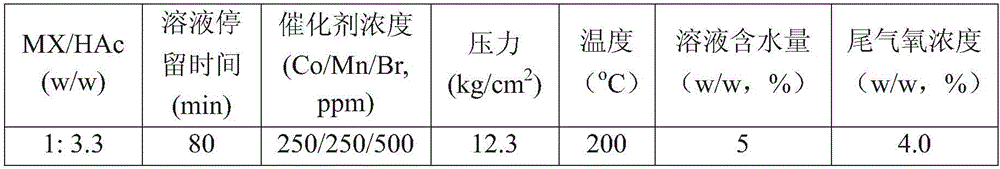
[0077] Such as figure 1 As shown, a typical PX production unit uses naphtha reforming method to produce aromatics and adsorption method to separate PX from C8 aromatics. The main process includes two modules: adsorption separation and isomerization. Containing the C 8 aromatics mixture S100 of the composition shown in Table 1 and the equilibrium stream S104 output by the isomerization unit, it is mixed and sent to the PX adsorption unit U101 together, and after adsorption and separation, the PX product with a purity of 99.5% is obtained, which is produced by the stream S101 The output system, the raffinate S102 is sent to the isomerization reactor U102 to conv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com