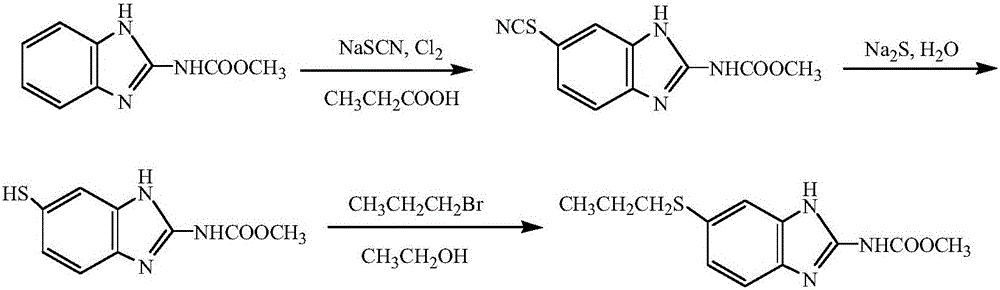
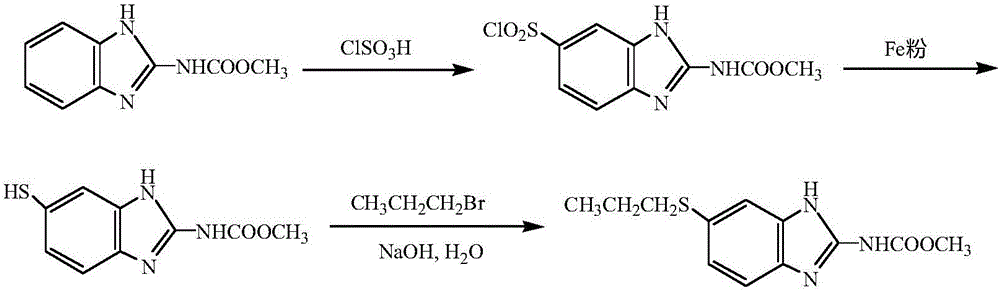
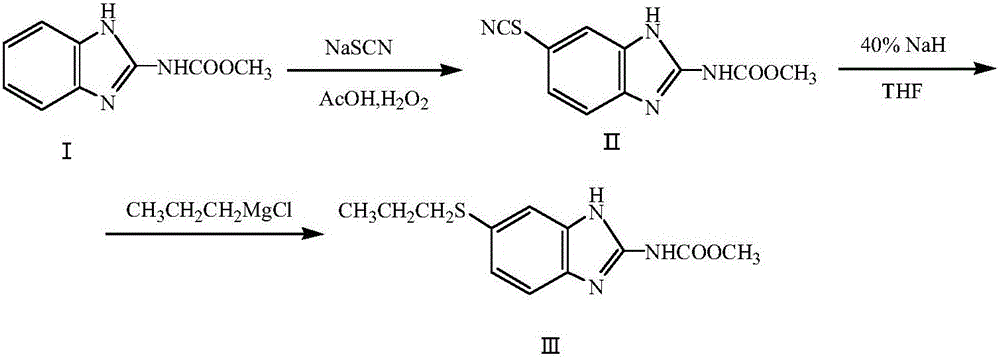
Method for preparing albendazole with chloropropane in place of bromopropane
A technology of albendazole and chloropropane, applied in the field of synthesis of imidazole compounds, can solve the problems of poor safety, low yield, high production cost, etc., and achieve the effects of safe production process, reduced environmental hazards, and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Add 100.00 g (0.52 mol) of carbendazim, 46.98 g (0.58 mol) of sodium thiocyanate, and 500.00 g of glacial acetic acid in a 1500 mL reaction flask in sequence, and slowly raise the temperature to 35° C. 253.00 g (2.08 mol) of hydrogen peroxide with a mass concentration of 28% was slowly added dropwise under the pressure, and the temperature in the reaction bottle was controlled at 35-37° C. After the hydrogen peroxide is added dropwise, continue to stir at 35°C and keep it warm for 6 hours. After the heat preservation is over, the solid is distilled under reduced pressure to obtain methyl 5-thiocyanobenzimidazole-2-carbamate, and the liquid glacial acetic acid is distilled out. recycle and re-use. After the glacial acetic acid has been distilled, add 1.50 g of benzyltriethylammonium chloride to the solid methyl 5-thiocyanobenzimidazole-2-carbamate, and slowly add 40% hydrogenated ammonium chloride in a stirring state. 110.00 g (1.82 mol) of sodium tetrahydrof...
Embodiment 2
[0022] Example 2: Add 100.00 g (0.52 mol) of carbendazim, 51.03 g (0.63 mol) of sodium thiocyanate, and 600.00 g of glacial acetic acid in a 1500 mL reaction flask in sequence, and slowly raise the temperature to 35° C. 253.00 g (2.08 mol) of hydrogen peroxide with a mass concentration of 28% was slowly added dropwise under the pressure, and the temperature in the reaction bottle was controlled at 35-37° C. After the hydrogen peroxide is added dropwise, continue to stir at 35°C and keep it warm for 4.5 hours. After the heat preservation is over, the solid is distilled under reduced pressure to obtain methyl 5-thiocyanobenzimidazole-2-carbamate, and the liquid ice is distilled off. Acetic acid recycling. After the glacial acetic acid has been distilled, add 2.0 g of benzyltriethylammonium chloride to the solid methyl 5-thiocyanobenzimidazole-2-carbamate, and slowly add 40% hydrogenated ammonium chloride in a stirring state. 124.80 g (2.08 mol) of sodium tetrahydrofuran solutio...
Embodiment 3
[0023]Example 3: 100.00g (0.52mol) of carbendazim, 59.13g (0.73mol) of sodium thiocyanate, and 500.00g of glacial acetic acid were sequentially added to a 1500mL reaction flask, and the temperature was slowly raised to 35°C. 253.00 g (2.08 mol) of hydrogen peroxide with a mass concentration of 28% was slowly added dropwise under the pressure, and the temperature in the reaction bottle was controlled at 35-37° C. After the hydrogen peroxide is added dropwise, continue to stir at 35°C and keep warm for 5 hours. After the keep warm, the solid is distilled under reduced pressure to obtain methyl 5-thiocyanobenzimidazole-2-carbamate, and the liquid glacial acetic acid is distilled out. recycle and re-use. After the glacial acetic acid has been distilled, add 2.00 g of benzyltriethylammonium chloride to the solid methyl 5-thiocyanobenzimidazole-2-carbamate, and slowly add 40% hydrogenated ammonium chloride in a stirring state. 140.40 g (2.34 mol) of sodium tetrahydrofuran solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com