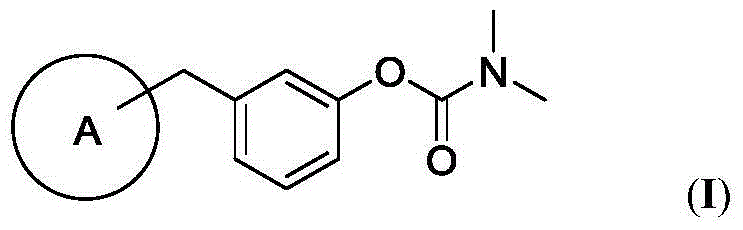
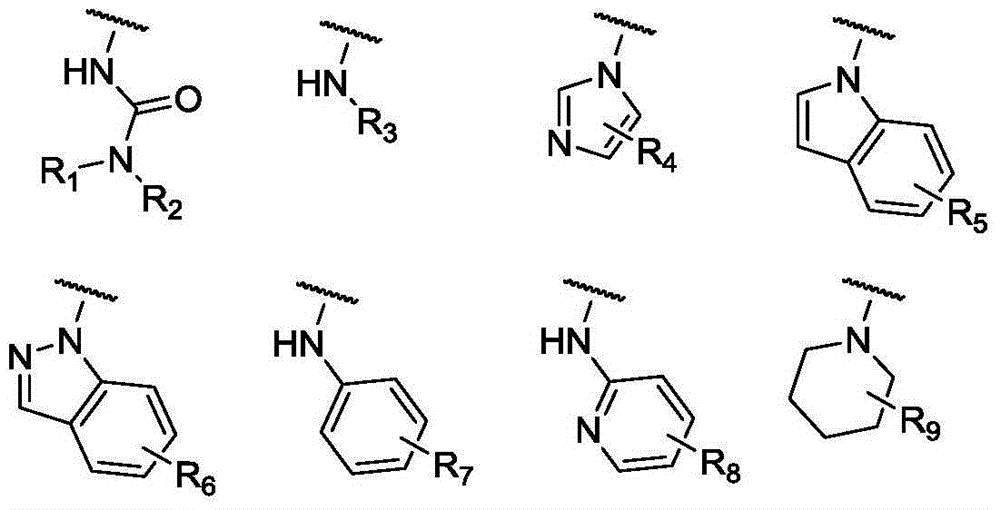
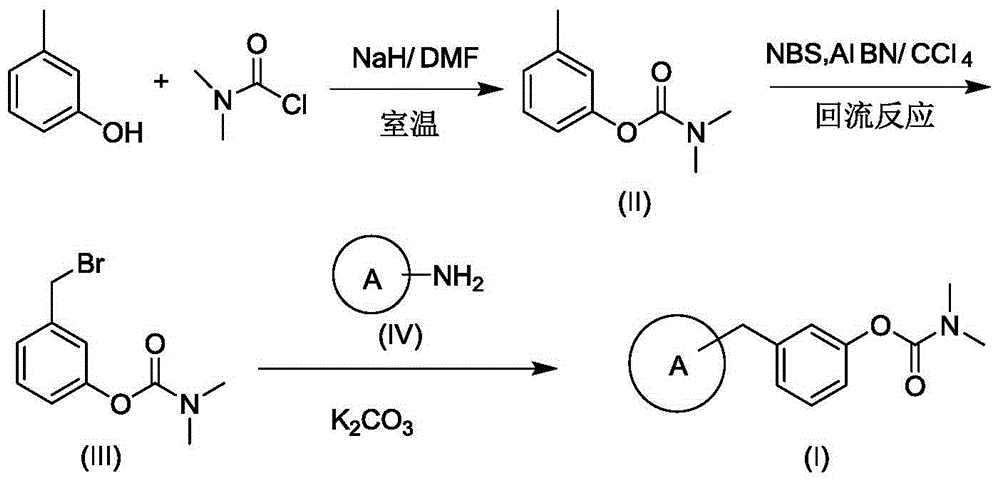
Carbamate derivative as well as synthetic method and application thereof
A carbamate and carbamate technology, applied in the field of carbamate derivatives and their synthesis, can solve the problems of low activity, short half-life, and large molecular weight of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Methyl 3-((1H-indazol-1-yl)methyl)phenyldimethylcarbamate (I 1 ) preparation
[0024] (1) Synthesis of m-tolyldimethylcarbamate
[0025] 4g of m-cresol was dissolved in 15mL of DMF, and 1.2g of sodium hydride was added. After stirring for 15 min, 4 g of dimethylcarbamoyl chloride were added dropwise. After reacting for 2 hours, 15 mL of water was added, and then extracted three times with 10 mL of ethyl acetate. After combining the three organic phases, the ethyl acetate was removed by rotary evaporation. Place it in a vacuum drying oven at 45° C. for 24 hours to obtain 5.9 g of a light pink transparent liquid product with a yield of 71.7%.
[0026] 1 H NMR (500MHz, CDCl 3 )δ7.25(t, J=7.8Hz, 1H, Ar-H), 7.02(d, J=7.6Hz, 1H, Ar-H), 6.93(m, J=13.6, 5.5Hz, 2H, Ar- H),3.12(s,3H,N-CH 3 ),3.03(s,3H,N-CH 3 ),2.37(s,3H,Ar-CH 3 ).
[0027] (2) Preparation of 3-(bromomethyl) phenyl dimethyl carbamate
[0028] Add 0.5g methyl m-tolyldimethylcarbamate, 0.593g bromosuccini...
Embodiment 2
[0034] 3-[(Imidazol-1-yl)methyl]phenyldimethylcarbamate (I 2 ) preparation
[0035]Dissolve 68 mg of imidazole in 5 mL of DMF, add 130 mg of potassium carbonate and 200 mg of 3-(bromomethyl)phenyldimethylcarbamate, and stir at room temperature. After the reaction was complete after about 4 hours, 5 mL of water was added and extracted three times with 10 mL of ethyl acetate. The organic phases were combined, and the ethyl acetate was distilled off under reduced pressure. Then, 5 mL of hydrochloric acid (1N) was added, and washed three times with 2 mL of ethyl acetate. Then the pH of the aqueous phase was adjusted to alkaline with anhydrous potassium carbonate, and then extracted three times with 5 mL of ethyl acetate, and the organic phases were combined and dried with anhydrous magnesium sulfate. Ethyl acetate was removed by rotary evaporation, and dried in a vacuum oven at 45°C to obtain 144 mg of a colorless transparent liquid with a yield of 76%.
[0036] 1 H NMR (500M...
Embodiment 3
[0038] Methyl 3-(anilinomethyl)phenyldimethylcarbamate (I 3 ) preparation
[0039] Dissolve 93 mg of aniline in 5 mL of DMF, add 130 mg of potassium carbonate and 200 mg of 3-(bromomethyl)phenyldimethylcarbamate, and stir at room temperature. After the reaction was complete after about 4 hours, 5 mL of water was added and extracted three times with 10 mL of ethyl acetate. The organic phases were combined, dried over anhydrous magnesium sulfate, ethyl acetate was distilled off under reduced pressure, separated by column chromatography, and dried in a vacuum oven at 45°C to obtain 141 mg of a yellow solid with a yield of 67%.
[0040] 1 H NMR (500MHz, CDCl 3 )δ7.35 (t, J=7.8Hz, 1H, Ar-H), 7.24–7.16 (m, 4H, Ar-H), 7.04 (m J=8.0, 1.8Hz, 1H, Ar-H), 6.77 –6.71(m,1H,Ar-H),6.65(m,J=11.6,4.0Hz,2H,Ar-H),4.36(s,2H,N-CH 2 -Ar),3.12(s,3H,N-CH 3 ),3.03(s,3H,N-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com