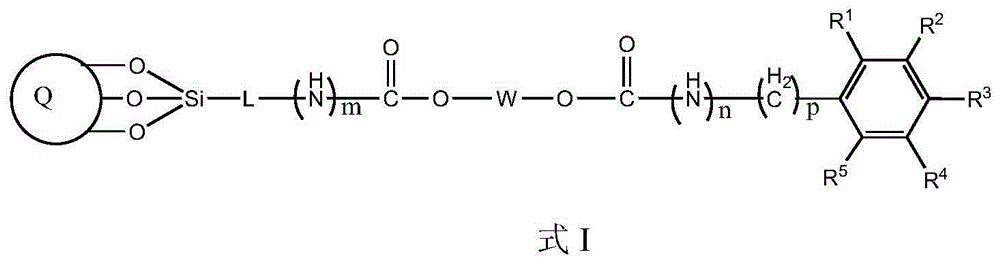
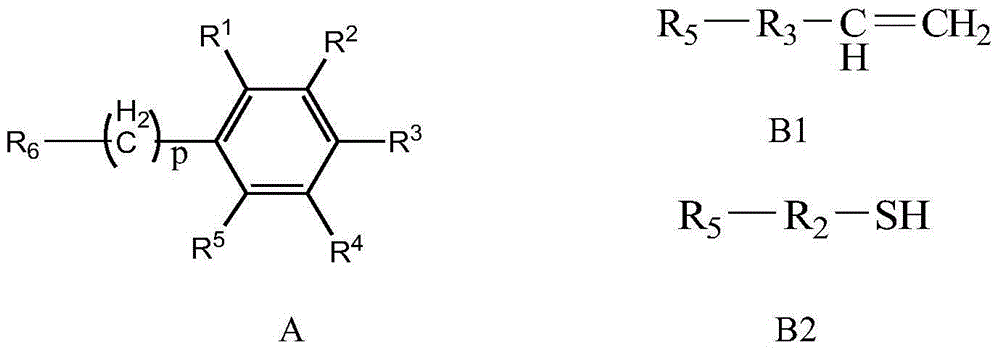
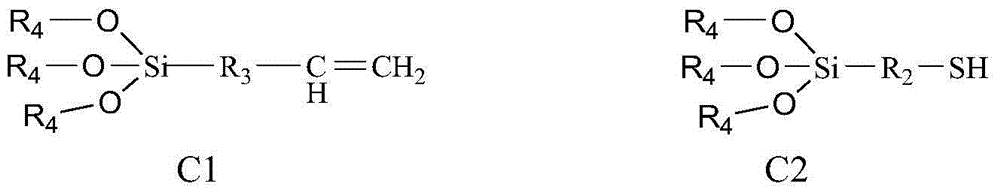Bonding polysaccharide chiral stationary phase and preparation method thereof
A chiral stationary phase and polysaccharide technology, applied in the field of chiral separation of liquid chromatography, can solve the problems of self-crosslinking, difficult regulation, and high requirements for experimental operation, and achieve long service life, strong solvent resistance, and stable structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of bonded starch benzoate chiral stationary phase
[0037] Take 1 g of starch (amylopectin extracted from corn), add it to a three-necked bottle filled with 10 g of 1-butyl-3-methylimidazolium chloride (BmimCl) ionic liquid, and stir mechanically at 60 °C for 2 hours to obtain a starch / ionic liquid solution . Add 5mL of benzoyl chloride and 0.1mL of acryloyl chloride at the same time, and react for 8 hours. After the reaction system is cooled to room temperature, pour the reaction solution into a large amount of isopropanol to precipitate, separate by filtration, collect the solid, and dry to obtain the bonded starch benzoate derivatives.
[0038] 5 g of pure silica (5 μm, ), dispersed in 50mL of toluene, added 1mL of pyridine, and added 3mL of γ-mercaptopropyltriethoxysilane at the same time, and reacted at 80°C for 12 hours. After the reaction system was cooled to room temperature, it was separated by filtration, and the solid was collecte...
Embodiment 2
[0039] Coat the prepared starch derivatives at 40% (w / w) on the mercapto-containing silica matrix, place it in a 50mL round bottom flask, add 30mL pyridine, 80mg tert-butyl hydroperoxide, 60°C React for 8 hours. After the reaction system is cooled to room temperature, it is separated by filtration, and the solid is collected, and then the solid is thoroughly washed with tetrahydrofuran and methanol, and dried in vacuum. The weight of the obtained product increases, which proves that the starch derivative has been bonded to the carbon dioxide On the silicon substrate, that is to say, a bonded starch benzoate chiral stationary phase was obtained. Example 2: Preparation of bonded cellulose phenyl carbamate chiral stationary phase
[0040] Take 1 g of cellulose (polymerization degree 650), add it into a three-necked flask filled with 20 g of 1-allyl-3-methylimidazolium chloride (AmimCl) ionic liquid, and mechanically stir at 80 ° C for 2 h to obtain a cellulose / ionic liquid solut...
example 3
[0043] Example 3: Preparation of bonded chitosan phenyl carbamate chiral stationary phase
[0044] Take 1g of chitosan (polymerization degree 600-1700), add it into a three-necked bottle filled with 20g of 1-ethyl-3-methylimidazole acetic acid (EmimAc) ionic liquid, and stir mechanically at 40°C for 2h to obtain chitosan / ion Liquid solution, add 0.5mL butylacryloyl chloride, react for 1h, then add 10mL phenylisocyanate, continue to react for 12 hours, wait for the reaction system to cool down to room temperature, pour the reaction solution into a large amount of ethanol, precipitate, separate by filtration, and collect the solid , and dried to obtain chitosan phenyl carbamate derivatives containing double bonds.
[0045] 5 g of pure silica (5 μm, ), dispersed in 50mL of toluene, added 2mL of pyridine, and simultaneously added 5mL of γ-mercaptopropyltrimethoxysilane, reacted at 90°C for 12 hours, cooled the reaction system to room temperature, separated by filtration, collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com