Preparation method of compound glycyrrhizin tablets and quality control detection method
A technology for glycyrrhizin tablets and glycyrrhizin, which is applied to the preparation process of compound glycyrrhizin tablets and the field of quality control and detection thereof, can solve the problems of complicated operation, inconvenient medication, many equipments and the like, so as to improve product quality and improve product quality. The effect of stability and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 3
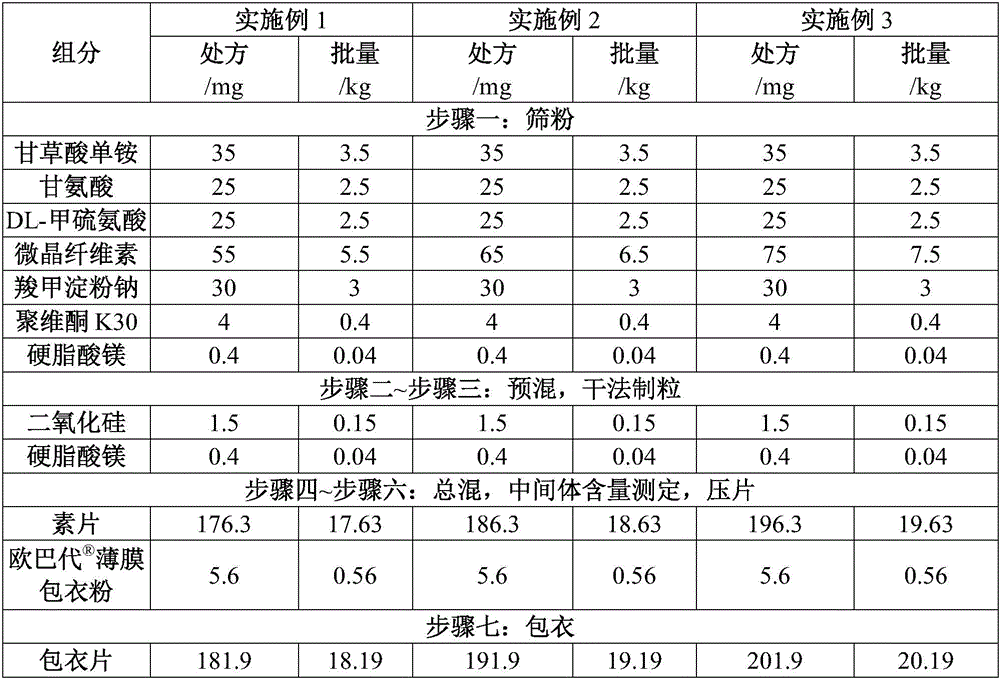
[0037] See Table 1 for the prescriptions and batches of the raw materials for the preparation of Compound Glycyrrhizin Tablets in Examples 1 to 3.
[0038] Table 1 The prescription and batch size of each preparation raw material for each glycyrrhizin tablet
[0039]
Embodiment 2
[0040] Taking Example 2 as an example, the specific preparation process of Compound Glycyrrhizin Tablets is as follows:
[0041] (1) Sieve powder: take monoammonium glycyrrhizinate, glycine, DL-methionine, diluent, disintegrant, silicon dioxide and magnesium stearate and pass through a 100-mesh sieve respectively, and set aside;
[0042] (2) Premixing: Weigh 3.5kg monoammonium glycyrrhizinate (2.5kg as glycyrrhizin), 2.5kg glycine, 2.5kg DL-methionine, 6.5kg microcrystalline fiber, 3.0kg carboxymethyl starch Add sodium, 0.4kg povidone K30, and 0.04kg magnesium stearate (half the amount of magnesium stearate) into the two-dimensional mixer, and mix for 20 minutes;
[0043] (3) Dry granulation: add the above-mentioned mixture into a dry granulator to granulate, and control the pressure to 4-5kg.
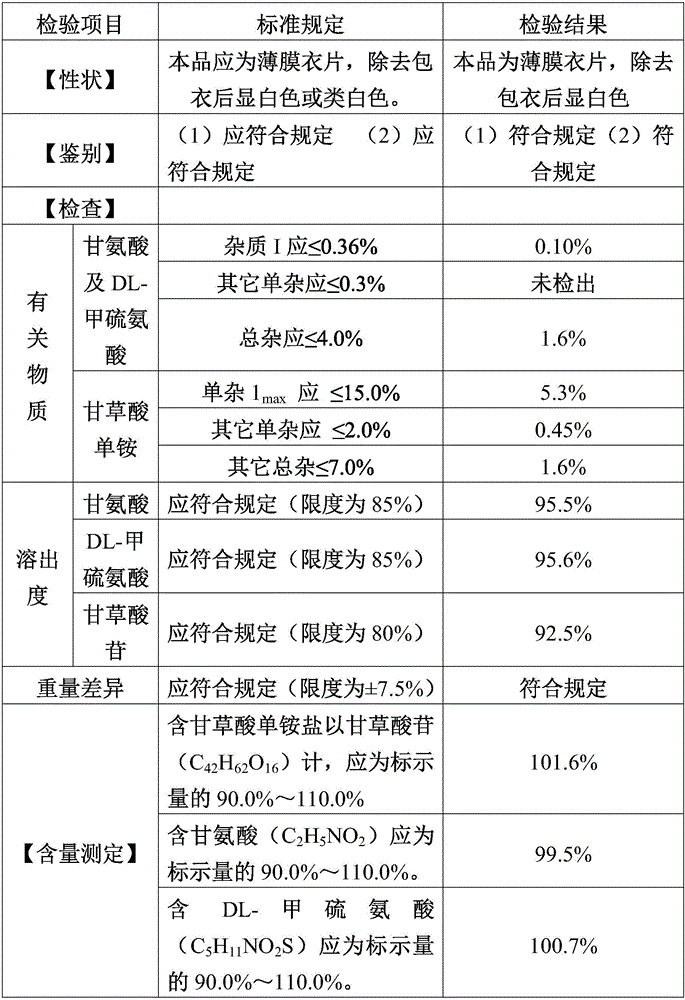
[0044] (4) Total blending: mix 0.15kg of silicon dioxide, 0.04kg of magnesium stearate (remaining magnesium stearate) with the above granules, and transfer the whole to a mixer for mixi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com