Method for synthesizing carbamate by ketone, amine and carbon dioxide
A carbamate and carbon dioxide technology, which is applied in the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of low substrate applicability, poor chemical selectivity, and low atom economy , to achieve the effects of reactive atom economy, simple operation and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
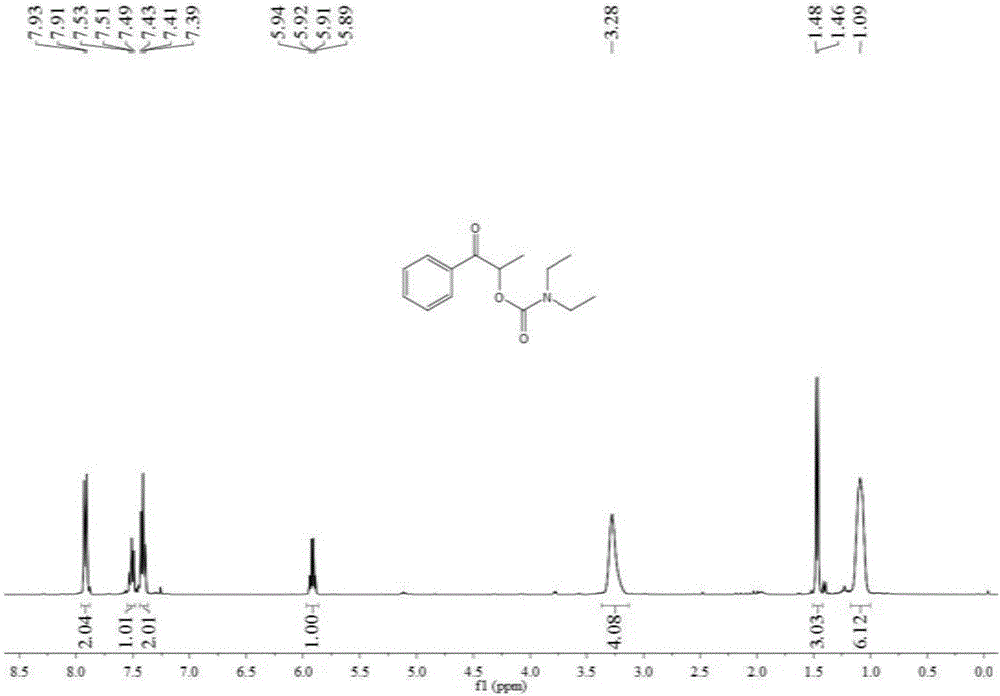
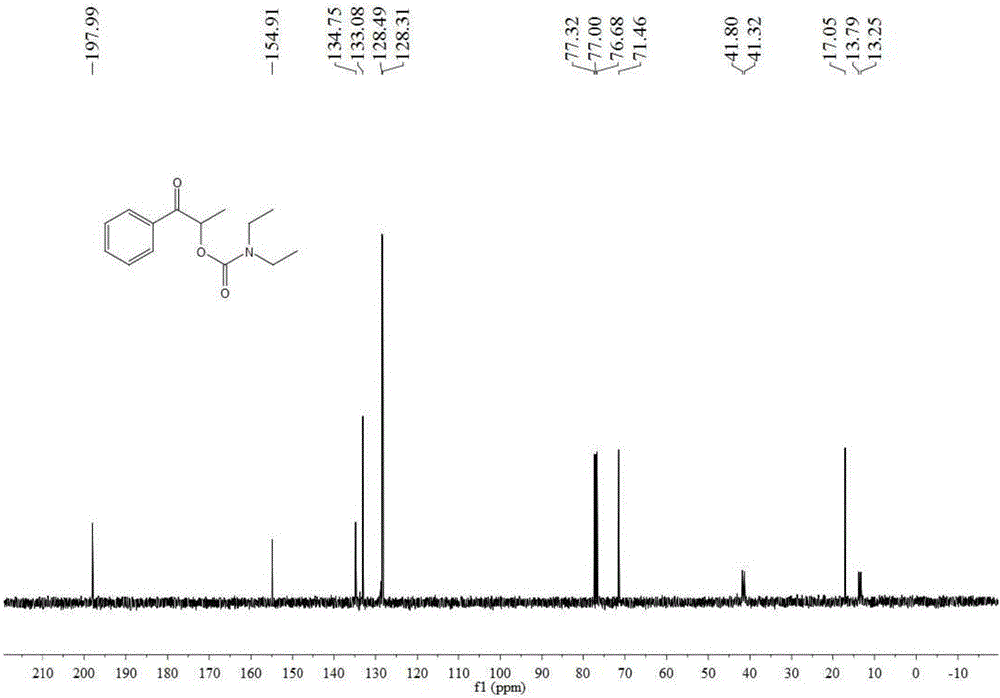
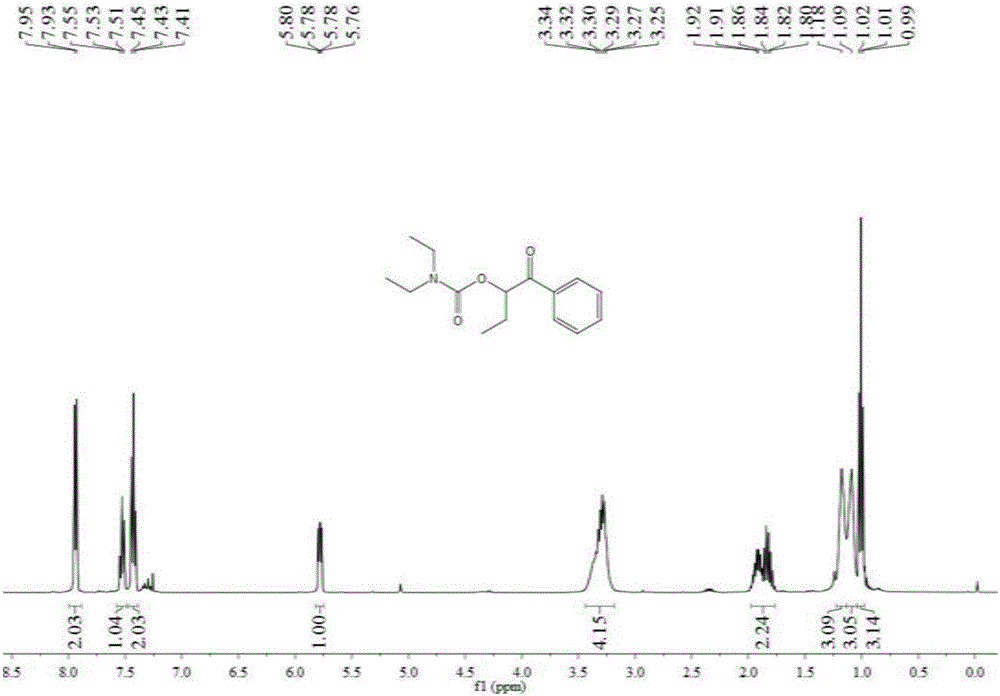
[0048] Add 1 mmol 1-phenyl-1-propanone, 0.2 mmol tetra-n-butylammonium iodide, 6 mmol tert-butanol peroxide, 3 ml dimethyl sulfoxide, 7 mmol Diethylamine, then slowly fill with carbon dioxide gas until its pressure reaches 3MPa, after stirring at 90°C for 12 hours, stop heating and stirring, cool to room temperature, and slowly release carbon dioxide to normal pressure. The reaction solution was washed with 20 mL of water, extracted three times with ethyl acetate (10 mL each time), the organic phases were combined and dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the yield was 60%.
Embodiment 2
[0050] Add 1 mmol of 1-phenyl-1-propanone, 0.2 mmol of tetra-n-butylammonium iodide, 8 mmol of tert-butanol peroxide, and 3 ml of N,N-dimethylformamide in the autoclave , 10 mmoles of diethylamine, and slowly filled with carbon dioxide gas until its pressure reached 3MPa, and stirred at 90°C for 6 hours, then stopped heating and stirring, cooled to room temperature, and slowly released carbon dioxide to normal pressure. The reaction solution was washed with 20 mL of water, extracted three times with ethyl acetate (10 mL each time), the organic phases were combined and dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the yield was 57%.
Embodiment 3
[0052] Add 1 mmol 1-phenyl-1-acetone, 0.2 mmol tetra-n-butylammonium iodide, 6 mmol tert-butanol peroxide, 3 milliliters of acetonitrile, and 7 mmoles of diethylamine in the autoclave, Slowly fill in carbon dioxide gas until its pressure reaches 3MPa, and after stirring at 90°C for 12 hours, stop heating and stirring, cool to room temperature, and slowly release carbon dioxide to normal pressure. The reaction solution was washed with 20 mL of water, extracted three times with ethyl acetate (10 mL each time), the organic phases were combined and dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 20:1, and the yield was 32%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com