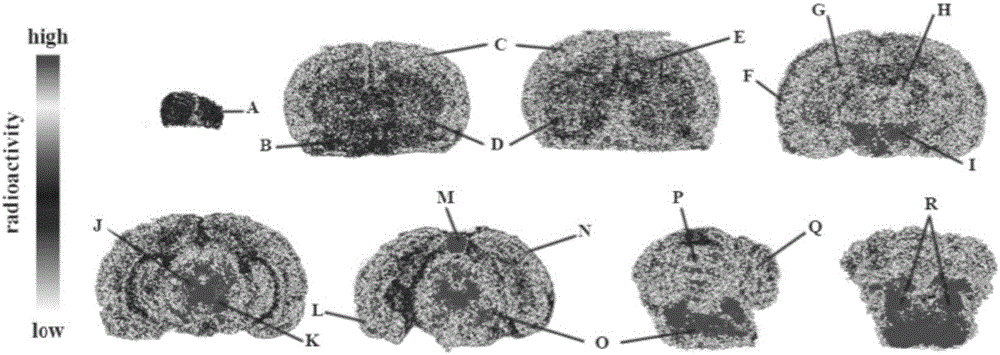
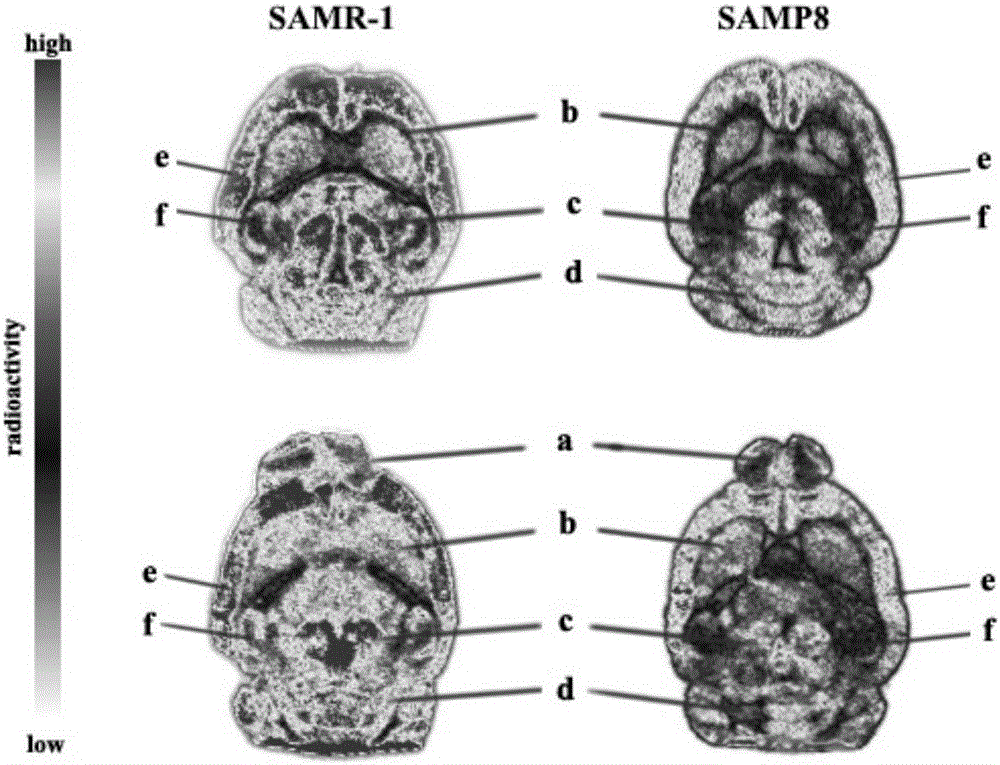
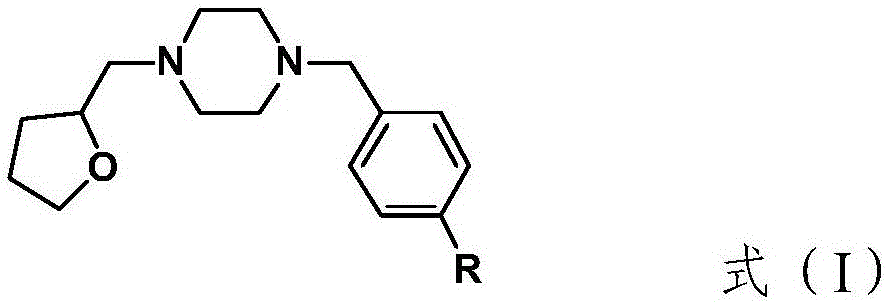
Tetrahydroxy furfuryl piperazine compound bonded with sigma-1 receptor and preparation method and application of compound
A compound, tetrahydroxyl technology, applied in the fields of radiopharmaceutical chemistry and nuclear medicine, can solve problems such as transportation restrictions and development constraints, and achieve the effects of high labeling rate, broad clinical application prospects, and high radiochemical purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Combining σ 1 Synthesis of Tetrahydroxyfurfurylpiperazines as Acceptors (R is F)
[0030] Tetrahydroxyfurfurylpiperazine (227mg, 1.33mmol), p-fluorobenzyl bromide (208mg, 1.10mmol), K 2 CO 3 (190mg, 1.37mmol), a catalytic amount (1-2mg) of KI was dissolved in CH 3 In CN (35mL), stir and reflux overnight at 80°C, filter and spin off acetonitrile, purify through a 200-300 mesh silica gel column, use methanol:dichloromethane=1:20 (volume ratio) as eluent, and obtain 203mg of light yellow liquid (Yield 66%). The structure of the product was detected by nuclear magnetic resonance analysis and high-resolution mass spectrometry, and the results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.33–7.20(m,2H),6.97(t,J=8.7Hz,2H),4.07–3.97(m,1H),3.90–3.81(m,1H),3.79–3.68(m,1H) ,3.45(s,2H),2.67–2.37(m,10H),2.03–1.91(m,1H),1.89–1.77(m,2H),1.52–1.41(m,1H). 13 C NMR (100MHz, CDCl 3 )δ161.94(d,J C-F =243Hz), 130.60, 114.90, 109.94, 77.17, 68.08, 63.31, 62.16, 53.70, 52.79,...
Embodiment 2
[0033] Example 2 Combining σ 1 Synthesis of tetrahydroxyfurfurylpiperazines as acceptors (R is -OCH 2 CH 2 F)
[0034] P-fluorooxyethylbenzyl bromide (260mg, 1.12mmol), tetrahydroxyfurfurylpiperazine (330mg, 1.34mmol) and K 2 CO 3 (231mg, 1.67mmol), a catalytic amount (1-2mg) of KI was dissolved in CH 3 In CN (35mL), stir and reflux overnight at 80°C, filter and spin off acetonitrile, purify through a 200-300 mesh silica gel column, use methanol:dichloromethane=1:20 (volume ratio) as eluent, and obtain 300mg of light yellow liquid (Yield 83%). The structure of the product was detected by nuclear magnetic resonance analysis and high-resolution mass spectrometry, and the results are as follows: 1 HNMR (400MHz, CDCl 3 )δ7.17(d, J=8.5Hz, 2H), 6.79(d, J=8.5Hz, 2H), 4.67(dt, J=47.5, 4.1Hz, 2H), 4.11(dt, J=28.0, 4.1 Hz,2H),4.04–3.95(m,1H),3.83–3.74(m,1H),3.70–3.61(m,1H),3.43(s,2H),2.73–2.27(m,10H),1.98– 1.85(m,1H),1.83–1.72(m,2H),1.49–1.33(m,1H). 13 C NMR (100MHz, CDCl 3 )...
Embodiment 3
[0037] Example 3 Combining σ 1 Synthesis of Acceptor Tetrahydroxyfurfurylpiperazines (R is I)
[0038] P-Iodobromobenzyl (234mg, 0.78mmol), tetrahydroxyfurfurylpiperazine (111mg, 0.65mmol), K 2 CO 3 (107mg, 0.65mmol) catalytic amount (1-2mg) of KI dissolved in CH 3 In CN (35mL), stir and reflux overnight at 80°C, filter and spin off acetonitrile, purify through a 200-300 mesh silica gel column, use methanol:dichloromethane=1:30 (volume ratio) as eluent, and obtain 259 mg of light yellow solid (Yield 63%). The structure of the product was detected by nuclear magnetic resonance analysis and high-resolution mass spectrometry, and the results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.55(d,J=8.2Hz,2H),7.00(d,J=8.2Hz,2H),4.07–3.97(m,1H),3.84–3.77(m,1H),3.73–3.63(m ,1H),3.39(s,2H),2.70–2.38(m,10H),2.00–1.89(m,1H),1.87–1.73(m,2H),1.48–1.34(m,1H). 13 C NMR (100MHz, CDCl 3 )δ137.55, 137.23, 131.08, 92.45, 76.11, 68.15, 62.91, 62.09, 53.45, 52.32, 30.23, 25.27. ESI-MS: [M+H] +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com