Protein for degrading patulin and encoding gene and application of protein
A patulin and coding technology, applied in the field of microorganisms, can solve the problems such as the effect is not very ideal, and achieve the effect of good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
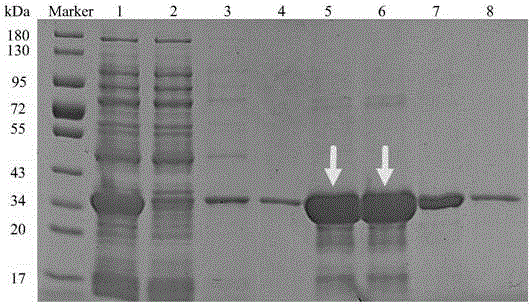
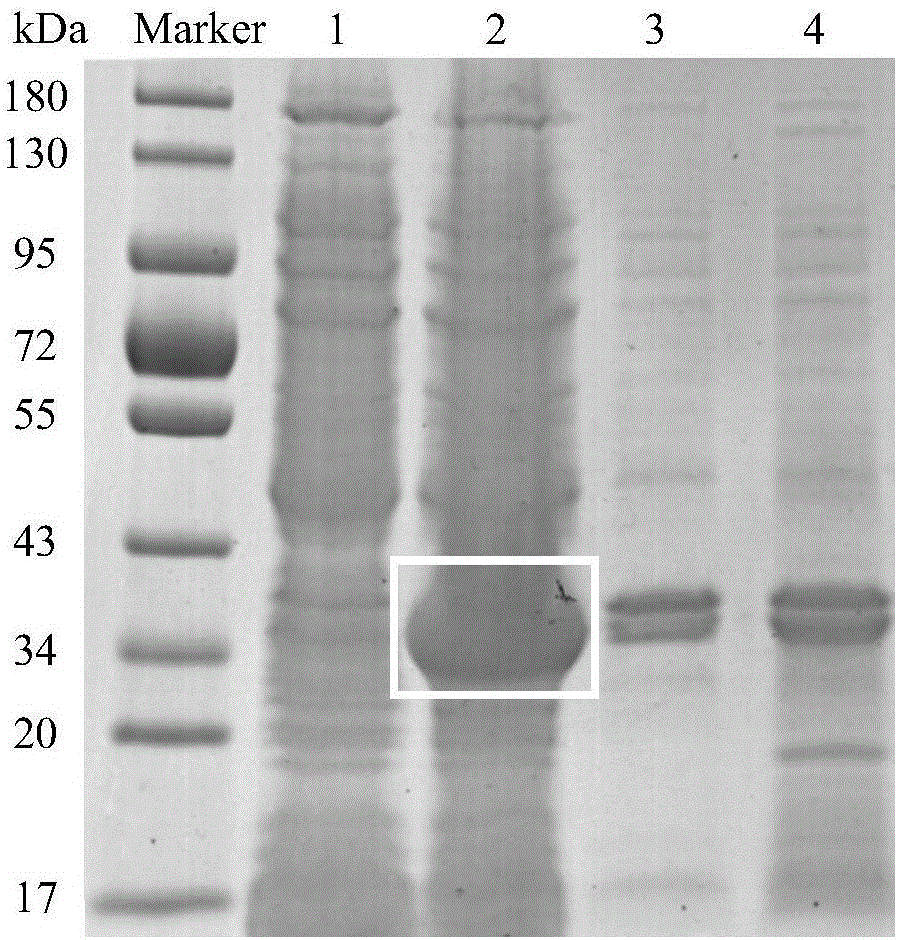
[0046] Cloning of embodiment 1, Cgscd gene and prokaryotic expression of Cgscd protein
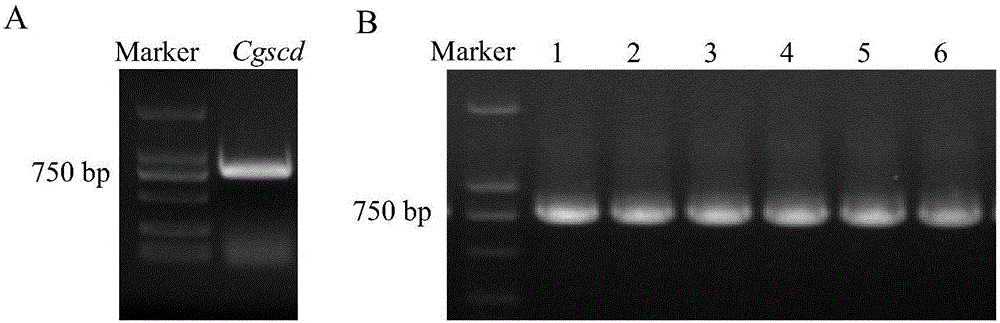
[0047] 1. Cloning of Cgscd gene
[0048] According to the GI number of Cgscd protein (gi|190348612, Short-chain dehydrogenase), look up the corresponding Cgscd gene sequence in NCBI, design primers at both ends of the gene and add BamHI and XhoI restriction sites to the primers, and name them respectively are pET-30a-F(BamHI) and pET-30a-R(XhoI).
[0049] pET-30a-F(BamHI): 5'- GGATCC ATGGAACAAACGTACTTTTATTTCAGGCG-3';
[0050] pET-30a-R(XhoI): 5'- CTCGAG TTACCATGGAAGTTCGGTTCCATCG-3'.
[0051]Candida guilliermondii (Candida guilliermondii) CGMCC 2.63 (recorded in "Yuanyuan Zong, Jia Liu, Boqiang Li, et al. Effects of yeast antagonists in combination with hot water treatment on postharvest diseases of tomato fruit. Biological Control 54 (2010) 316 -321 "article, the public can obtain from the applicant, can only be used for repeating the genomic DNA of the experiment of the present inv...
Embodiment 2
[0076] Example 2, Cgscd protein degradation research on patulin
[0077] After the concentration of the purified Cgscd protein in Example 1 was measured by the Coomassie brilliant blue method, the purified protein was taken to explore the effects of different coenzymes, pH and co-cultivation temperature on the catalytic degradation of patulin in vitro by the Cgscd protein.
[0078] Patulin: Canada Toronto Research Chemicals (TRC) company product, its catalog number is P206500.
[0079] 1. Coenzyme
[0080] It is divided into 4 groups: (1) reduced coenzyme Ⅰ (NADH); (2) oxidized coenzyme Ⅰ (NADH) + ); (3) reduced coenzyme Ⅱ (NADPH); (4) oxidized coenzyme Ⅱ (NADP + ).
[0081]The total volume of the reaction system is 300 μL, which contains 245 μL of 50 mM MES buffer solution (pH6) (preparation method: MES is dissolved in an appropriate amount of water, and the pH is adjusted to 6 with 2M NaOH, and then the water is made to volume so that the final concentration of MES in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com