Homocysteine (HCY) detection reagent with strong stability
A homocysteine and stable technology, applied in the field of homocysteine (HCY) detection reagents, can solve the problems of low detection reagents, high cost, and radioactive pollution, so as to improve precision and prevent corruption , the effect of preventing growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
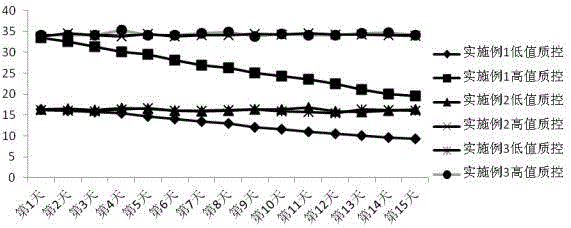
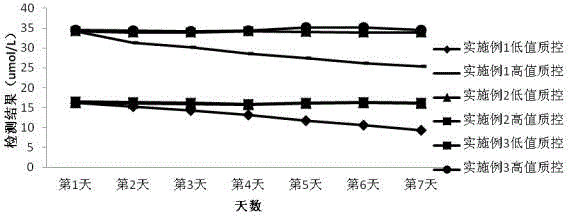
Image
Examples
Embodiment 1
[0055] A configuration scheme of an existing dual-reagent HCY detection reagent on the market:
[0056]R1: NADH (0.47 mM), LDH (38 KU / L), Serine (0.76 mM), Tris buffer (50mM), NaN3 (<1%), TCEP (2.9 mM);
[0057] R2: CBS (0.748 KU / L); CBL (16.4 KU / L) NaN3 (<1%).
[0058] The usage method of this embodiment reagent:
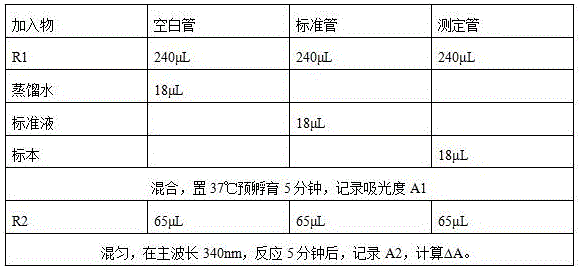
[0059] The HCY detection reagent described in this example is used with a fully automatic biochemical analyzer with dual reagent functions, such as Hitachi 7180 fully automatic analyzer, etc., and is determined by a fixed time method. Place R1 and R2 on the corresponding reagent positions according to the ratio of 48:13, and place distilled water, standards and samples on the corresponding positions of the sample tray. The operation is as follows: Figure 5 .
Embodiment 2
[0061] This example describes a highly stable liquid dual-reagent HCY detection reagent, consisting of:
[0062] Reagent 1 (R1):
[0063] TRIS Tris hydroxymethyl aminomethane 100mmol / L
[0064] NADH 0.47mmol / l
[0065] LDH 3KU / L
[0066] Serine 0.76mmol / L
[0067] MIT (Methylisothiazolinone) 0.5g / L
[0068] TCEP (trichloroethyl phosphate) 2.9mmol / L
[0069] Polyethylene glycol 400 2ml / L
[0070] Triton-405 1ml / L
[0071] Disodium EDTA 1mmol / L
[0072] CCD (Shanghai Xibao) 3g / L
[0073] Reagent 2 (R2):
[0074] TES (N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid) buffer 100mmol / L
[0075] MIT (Methylisothiazolinone) 0.5g / L
[0076] Ethylene glycol 3m / L
[0077] β-cyclodextrin 10g / L
[0078] Triton-405 1ml / L
[0079] Xanthan gum 0.5g / L
[0080] CBS 0.5 KU / L
[0081] CBL 3 KU / L.
[0082] The usage method of this embodiment reagent:
[0083] The HCY detection reagent described in this example is used with a fully automatic biochemical analyzer with dual r...
Embodiment 3
[0085] The reagent composition of a liquid double-reagent HCY detection reagent with strong stability after the key protection component is increased as described in this example:
[0086] Reagent 1 (R1):
[0087] TRIS Tris hydroxymethyl aminomethane 100mmol / L
[0088] NADH 0.47mmol / l
[0089] LDH 3KU / L
[0090] Serine 0.76mmol / L
[0091] MIT (Methylisothiazolinone) 1g / L
[0092] TCEP (trichloroethyl phosphate) 2.9mmol / L
[0093] Polyethylene glycol 400 5ml / L
[0094] Triton-405 2ml / L
[0095] Disodium EDTA 5mmol / L
[0096] CCD (Shanghai Xibao) 5g / L
[0097] Reagent 2 (R2):
[0098] TES (N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid) buffer 100mmol / L
[0099] MIT (Methylisothiazolinone) 1g / L
[0100] Ethylene glycol 6m / L
[0101] β-cyclodextrin 20g / L
[0102] Triton-405 2ml / L
[0103] Xanthan gum 1g / L
[0104] CBS 0.5KU / L
[0105] CBL 3KU / L.
[0106] The usage method of this embodiment reagent:
[0107] The HCY detection reagent described in this exa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com