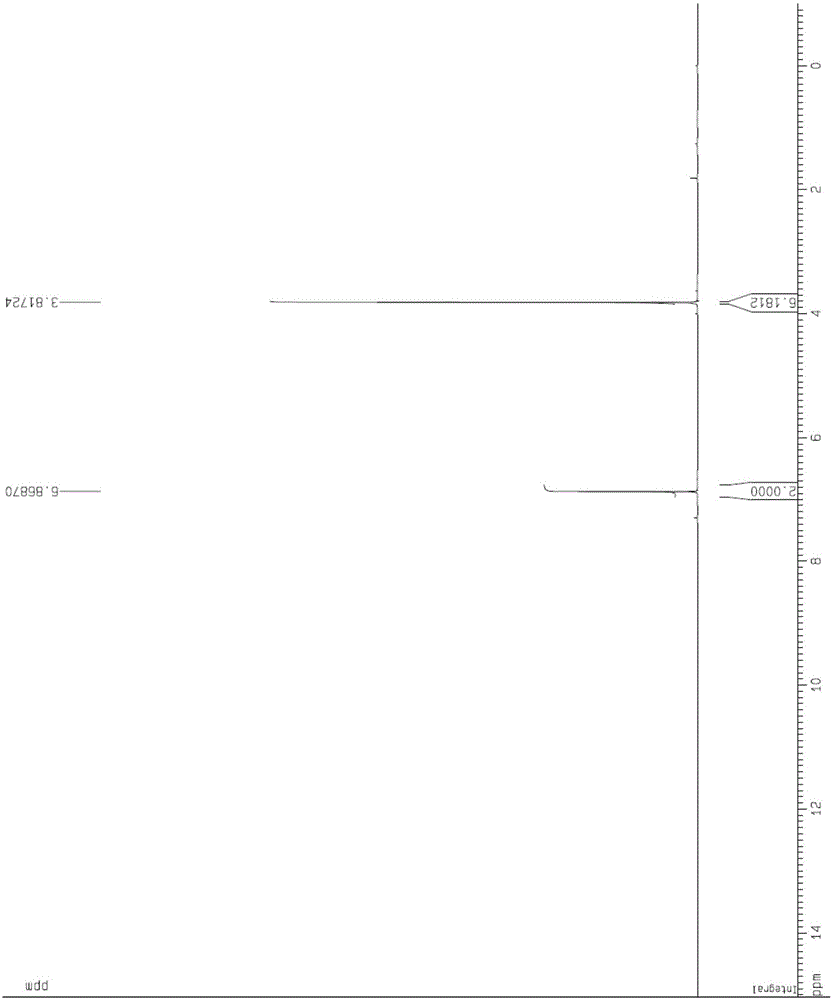Dimethyl fumarate preparation method
A technology of dimethyl fumarate and fumaric acid, applied in the field of chemistry, can solve problems such as irritating reactions, cumbersome steps, and great safety risks for workers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The invention discloses a method for preparing dimethyl fumarate, and those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
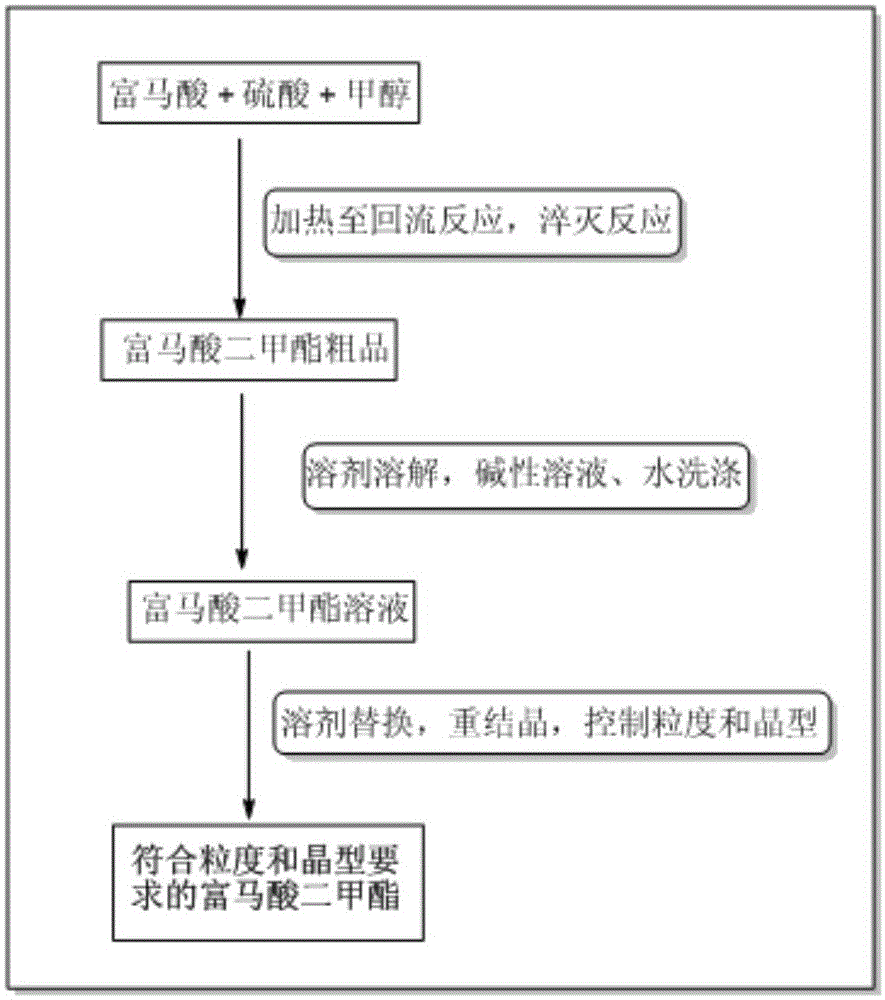
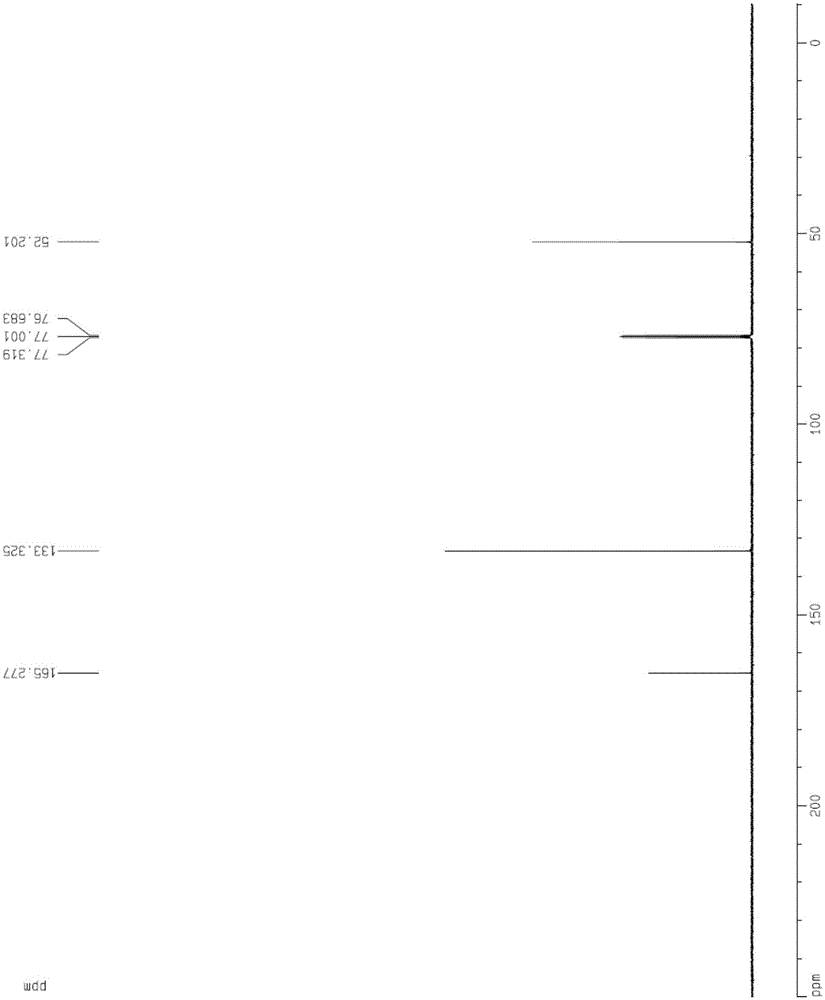
[0065] The content of the present invention is the post-treatment process of dimethyl fumarate and the control process of crystal form and particle size. Through the improvement of the process, the fu...
Embodiment 1
[0090] Embodiment 1: the synthesis of dimethyl fumarate crude product
[0091] Add 70L of methanol into a 100L reactor, slowly add 1.4kg of sulfuric acid, and stir at a speed of about 150±50 rpm; after the sulfuric acid methanol solution is prepared, add 14kg (mol) of fumaric acid, and heat to reflux for more than 8 hours. HPLC detects that raw material reaction is complete and can stop reaction. After the reaction is stopped, the reaction is pumped into a container containing 140L of ice water while it is hot. Stir continuously during the operation. As the temperature decreases, a large amount of white powder solids precipitate out. Continue to stir for 1 hour, and centrifuge with a 400-mesh filter cloth to obtain fuma Crude dimethyl ester. The crude product is subject to further purification.
[0092]
[0093] Synthesis of Crude Dimethyl Fumarate
Embodiment 2
[0094] Embodiment 2: post-treatment of dimethyl fumarate crude product
[0095] The crude dimethyl fumarate obtained in Example 1 was dissolved in 84L of ethyl acetate and then added to a 150L extraction kettle. Added 30L of saturated sodium bicarbonate solution and stirred for 10 minutes at a speed of 150±50 rpm. After stirring, let it stand for stratification, separate the lower aqueous phase, and continue to wash the upper organic phase twice with saturated sodium bicarbonate solution and once with saturated sodium chloride solution. The amount of washing solution is 30L / time to ensure that the pH of the aqueous phase solution is 7. . The organic phase was dried with 5 kg of anhydrous sodium sulfate for 30 minutes, and separated by filtration to obtain an ethyl acetate solution of dimethyl fumarate. The synthetic specific process of dimethyl fumarate crude product is as follows:
[0096]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com