Bilastine intermediate and preparation and purification methods therefor
A purification method and compound technology, applied in the direction of organic chemistry, etc., can solve problems such as unfavorable storage and transportation, difficulty in purification, etc., and achieve the effects of convenient transportation and storage, reduction of purification steps, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14-
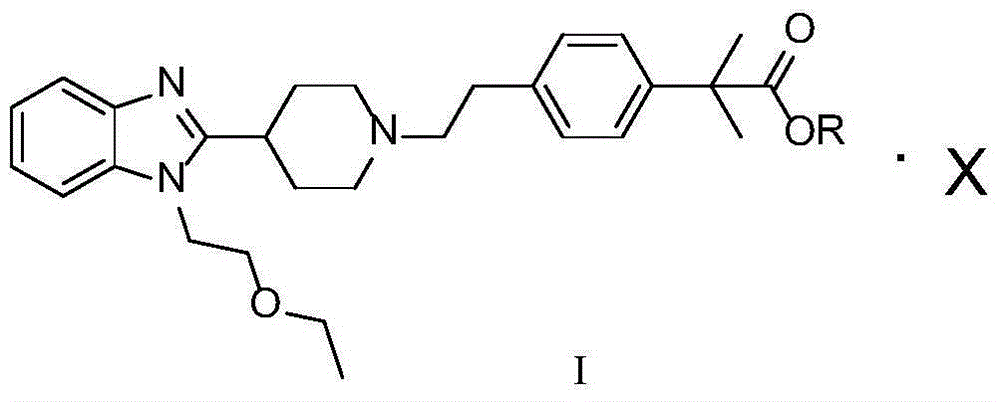
[0020] Example 14-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylbenzene Preparation and Purification of Ethyl Acetate Oxalate
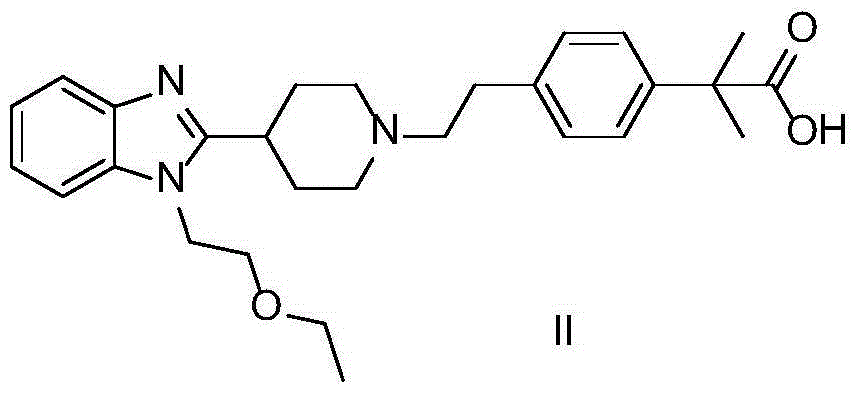
[0021] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid Add 10g of ethyl ester, add 80mL of acetonitrile, after the solid is dissolved, add 2.2g of oxalic acid, stir at room temperature for 5-7 hours, filter the product, wash with 20mL of acetonitrile, and dry under vacuum at 40°C to obtain 11.2g of white crystals with a yield of 92% . Melting point: 183°C-185°C. 1 H NMR(400MHz,DMSO),δ(ppm):1.08(t,3H),1.12(t,3H),1.51(s,6H),1.46-1.89(m,4H),1.92-2.14(m,2H ),2.54-2.57(m,2H),2.73-2.77(m,2H),3.03-3.09(m,3H),3.04-3.09(q,2H),3.11-3.16(q,2H),3.64-3.67 (t,2H),4.38-4.41(t,2H),7.14-7.21(m,4H),7.26-7.38(m,2H),7.51-7.57(dd,2H).H RMS(EI):m / z calcd.for C 32 h 43 N 3 o 7 :581.3101.Found:581.3102.
[0022] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidin...
Embodiment 24-
[0023] Example 24-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylbenzene Preparation and Purification of Ethyl Acetate Oxalate
[0024] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid Add 10g of ethyl ester, add 80mL of ethanol, after the solid is dissolved, add 2.2g of oxalic acid, stir at room temperature for 5-7 hours, filter the product, wash with 20mL of acetonitrile, and dry under vacuum at 40°C to obtain 10.7g of white crystals with a yield of 88% . Melting point: 183°C-185°C. 1 H NMR(400MHz,DMSO),δ(ppm):1.08(t,3H),1.12(t,3H),1.51(s,6H),1.46-1.89(m,4H),1.92-2.14(m,2H ),2.54-2.57(m,2H),2.73-2.77(m,2H),3.03-3.09(m,3H),3.04-3.09(q,2H),3.11-3.16(q,2H),3.64-3.67 (t,2H),4.38-4.41(t,2H),7.14-7.21(m,4H),7.26-7.38(m,2H),7.51-7.57(dd,2H).H RMS(EI):m / z calcd.for C 32 h 43 N 3 o 7 :581.3101.Found:581.3102.
[0025] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]eth...
Embodiment 34-
[0026] Example 34-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylbenzene Preparation and Purification of Ethyl Acetate Hydrochloride
[0027] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimethylphenylacetic acid 10g of ethyl ester, add 80mL of methanol, after the solid dissolves, add excess HCl gas, stir at room temperature for 3-5 hours, filter the product, wash with 20mL of methanol, and dry under vacuum at 40°C to obtain 10.5g of white crystals, the yield 97%. Melting point: 168°C-170°C. 1 HNMR(400MHz,DMSO),δ(ppm):1.05(t,3H),1.10(t,3H),1.48(s,6H),1.50-1.87(m,4H),1.97-2.10(m,2H) ,2.55-2.59(m,2H),2.77-2.80(m,2H),2.99-3.04(m,3H),3.05-3.11(q,2H),3.13-3.17(q,2H),3.54-3.61( t,2H),4.31-4.36(t,2H),7.16-7.23(m,4H),7.27-7.39(m,2H),7.55-7.59(dd,2H).H RMS(EI):m / z calcd.for C 30 h 42 ClN 3 o 3 :527.2915. Found: 527.2915.
[0028] Take 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com