Tetraphenyl ethylene derivative ion complex and preparation method thereof
An ion complex and tetraphenylethylene technology, which is applied in the field of tetraphenylethylene derivative ion complex and preparation, can solve the problems of complex synthesis steps, low yield and the like, and achieves simple preparation method and high yield , the effect of obvious aggregation-induced luminescence effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of four-(4-hydroxyphenyl)ethylene (III), comprises the steps:
[0043] (1)N 2 Under protection, add 150ml of dry tetrahydrofuran into a 500ml two-neck flask, stir at room temperature for 5min, add zinc powder, slowly add titanium tetrachloride dropwise in ice bath, reflux at 75°C for 2h, cool to room temperature, add 20g of 4,4 '-Dimethoxybenzophenone (I), in N 2 During protection, react at 75°C for 24 hours to obtain mixture 2; the amount of zinc powder added is 33% of the mass of 4,4'-dimethoxybenzophenone (I); the addition of titanium tetrachloride The amount is 48% of the quality of 4,4'-dimethoxybenzophenone (I);
[0044] (2) With the potassium carbonate of mixture 2,20g, the deionized water of 20ml and the dichloromethane of 120ml, after fully mixing, vacuum filter with diatomaceous earth, after the obtained filtrate is rotary evaporated to remove solvent, the residue is used Dichloromethane and methanol were recrystallized to remove unre...
Embodiment 2
[0050] A preparation method of tetraphenylethylene derivative ion complex, comprising the steps of:
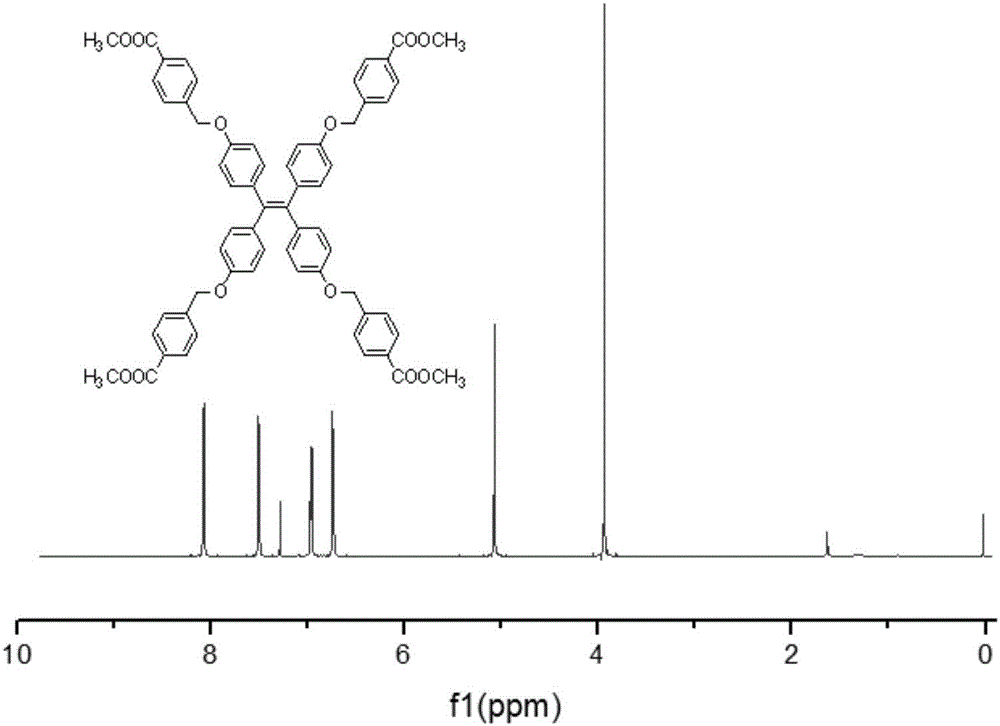
[0051] (1) Weigh 0.5g (1.26mmol) of tetra-(4-hydroxybenzene)ethylene (III), 1.16g of methyl 4-bromomethylbenzoate, dissolve with 20ml of acetone, add 0.7g of potassium carbonate and 122mg (0.379 mmol) phase transfer catalyst tetrabutylammonium bromide; heated to reflux for 18h to obtain mixture 1; acetone was removed by rotary evaporation, the residue was dissolved in dichloromethane, extracted three times with saturated aqueous sodium chloride solution, and the organic layer was collected with anhydrous Sodium sulfate is dried, and the dichloromethane solvent is removed by rotary evaporation of the dried organic solution, and the residue is recrystallized with dichloromethane / methanol, and a precipitate is precipitated, filtered and dried to obtain compound (IV), and the molecular weight of compound (IV) is 989.09 , whose structure was characterized by H NMR spectroscopy, see...
Embodiment 3
[0059] A preparation method of tetraphenylethylene derivative ion complex, comprising the steps of:
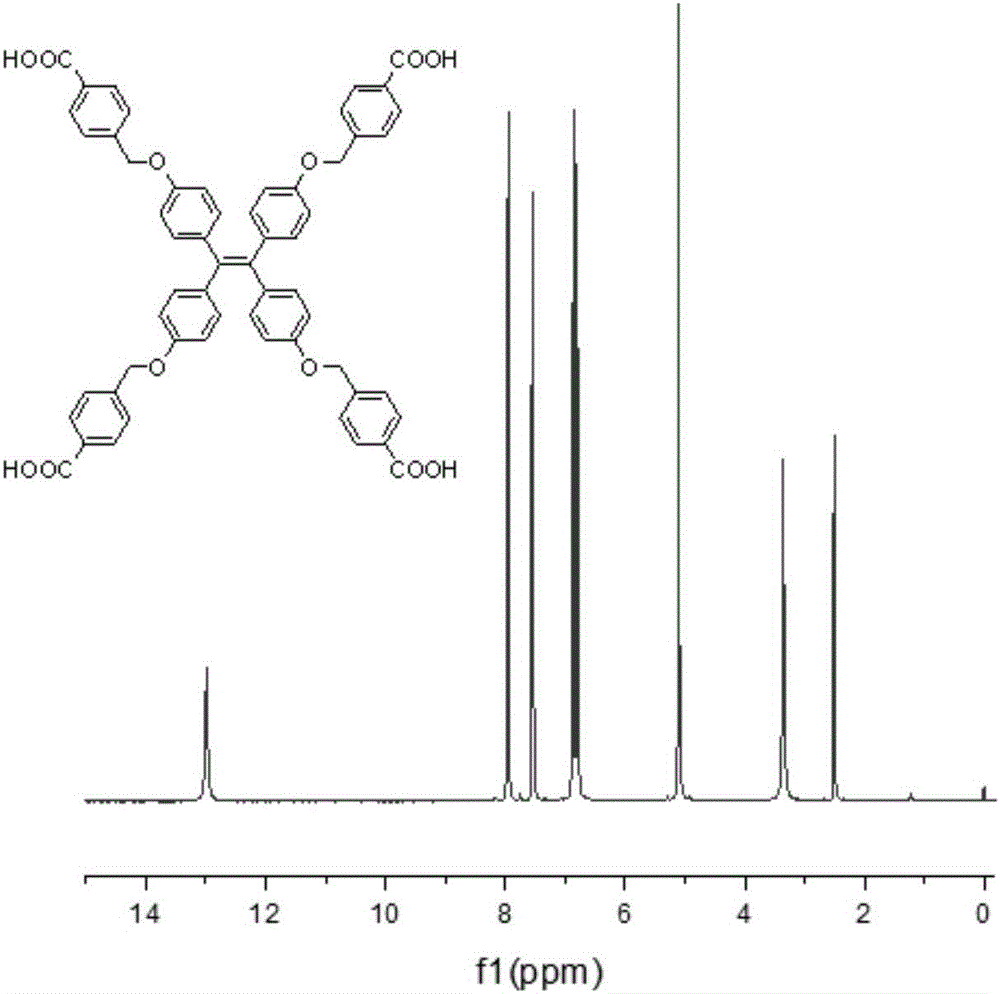
[0060] (1) Take tetra-(4-hydroxybenzene) ethylene (III) 0.5g (1.26mmol), methyl 4-bromomethylbenzoate 1.45g, dissolve with 20ml acetone, add 0.87g potassium carbonate and 0.2g ( 0.62mmol) phase-transfer catalyst tetrabutylammonium bromide; Heated to reflux for 24h to obtain mixture 1; Rotary evaporation removed acetone, the residue was dissolved in dichloromethane, and extracted three times with saturated aqueous sodium chloride solution, and the organic layer was collected and used without Water sodium sulfate is dried, and the dichloromethane solvent is removed by rotary evaporation of the dried organic solution; The residue is recrystallized with dichloromethane / methanol, and a precipitate is separated out, filtered and dried to obtain compound (IV), and the molecular weight of compound (IV) is 989.09;
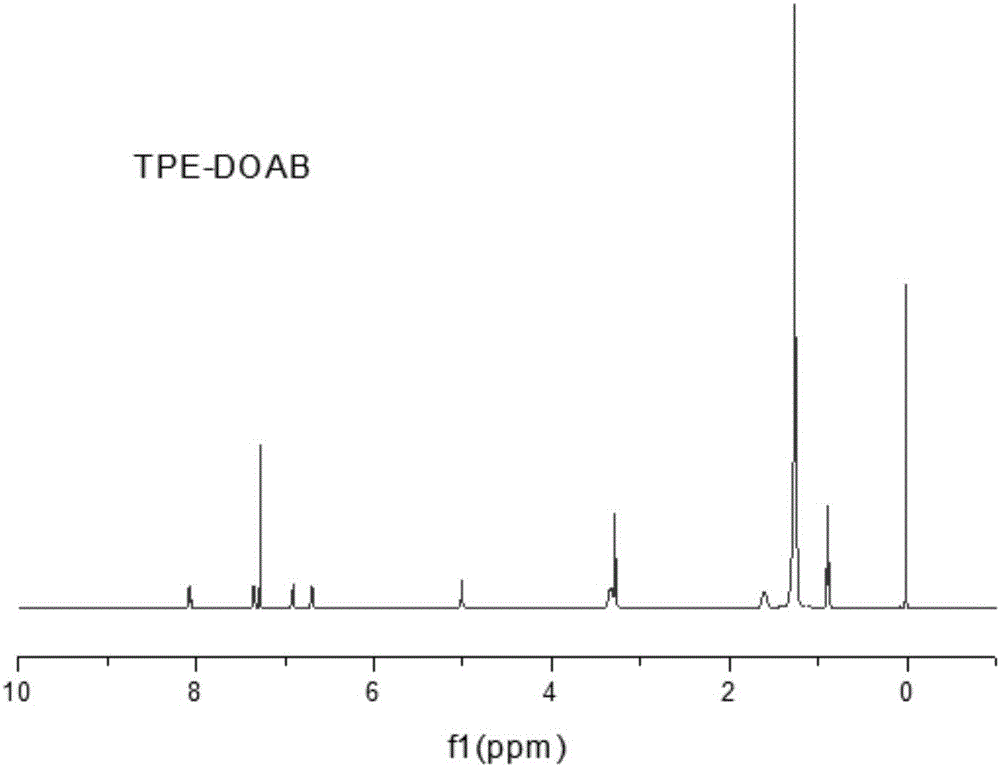
[0061] (2) Weigh 0.4g of compound (IV), heat and dissolve in 10mL tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com