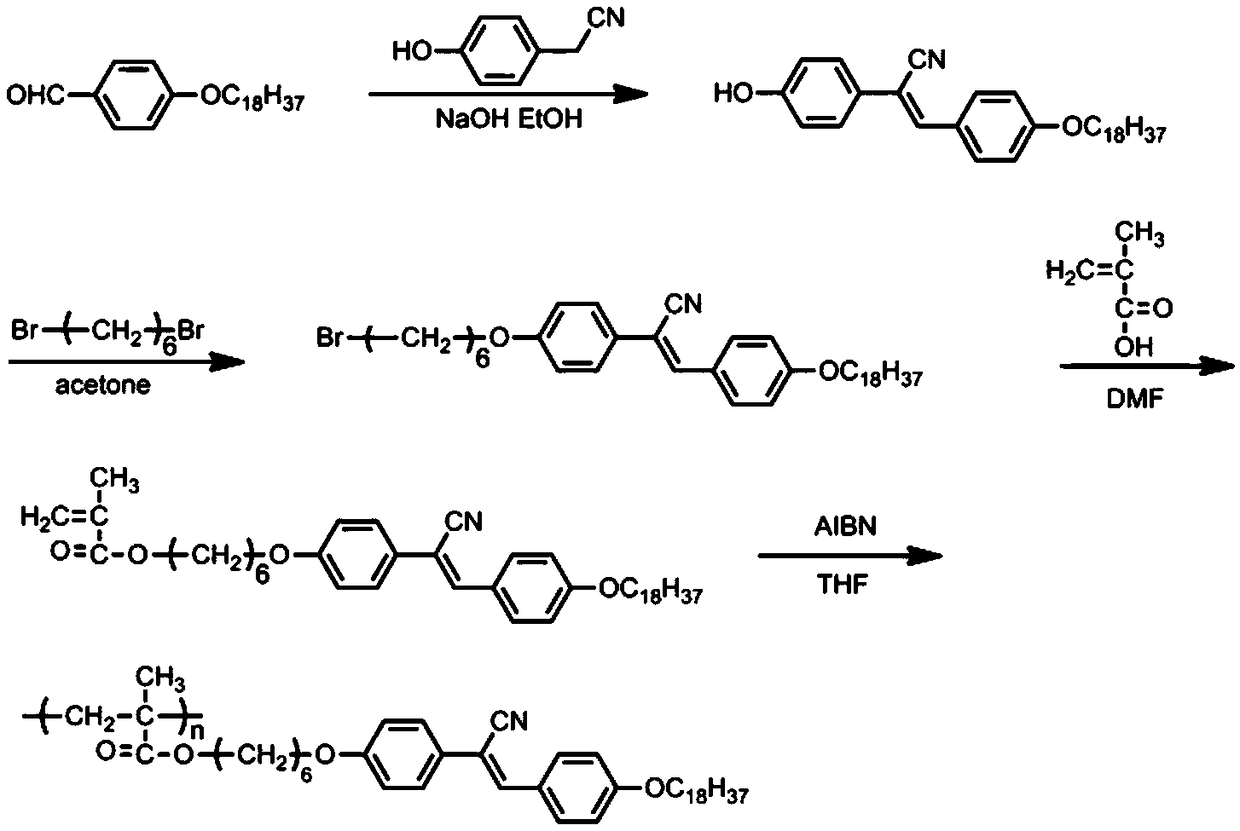
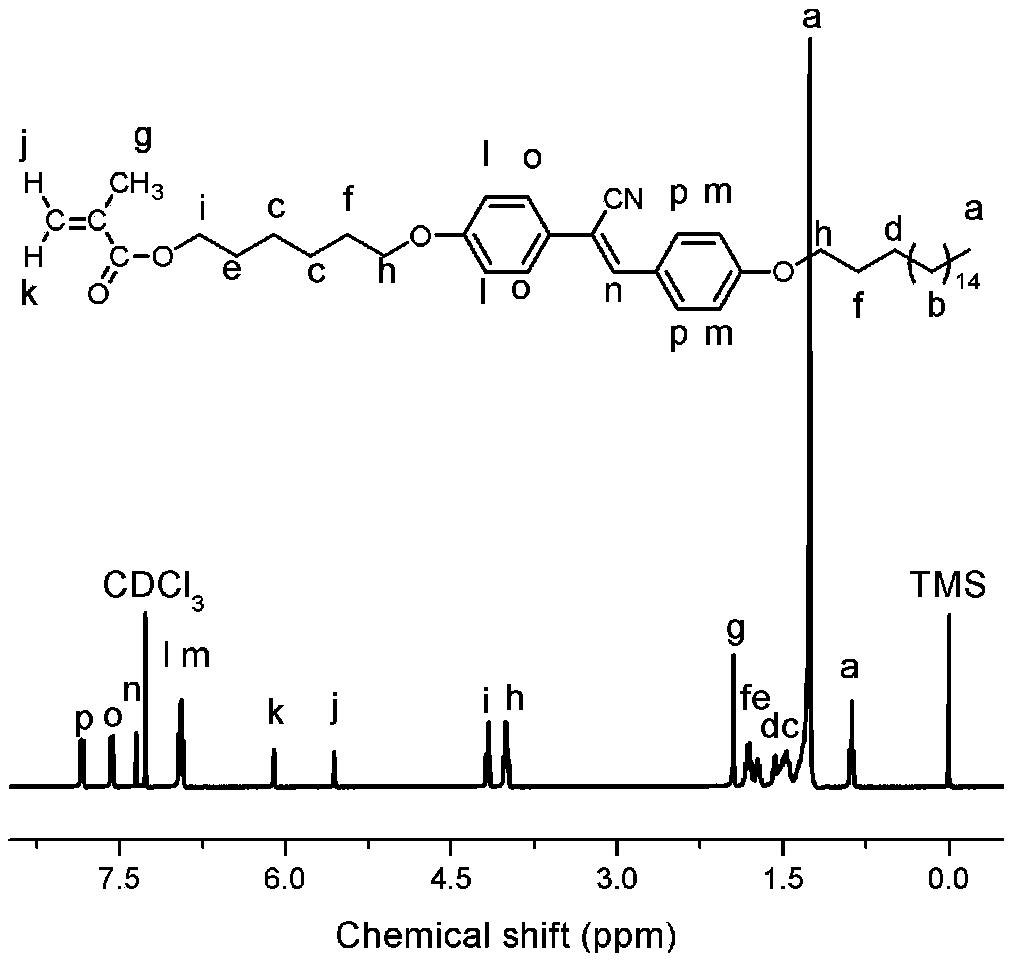
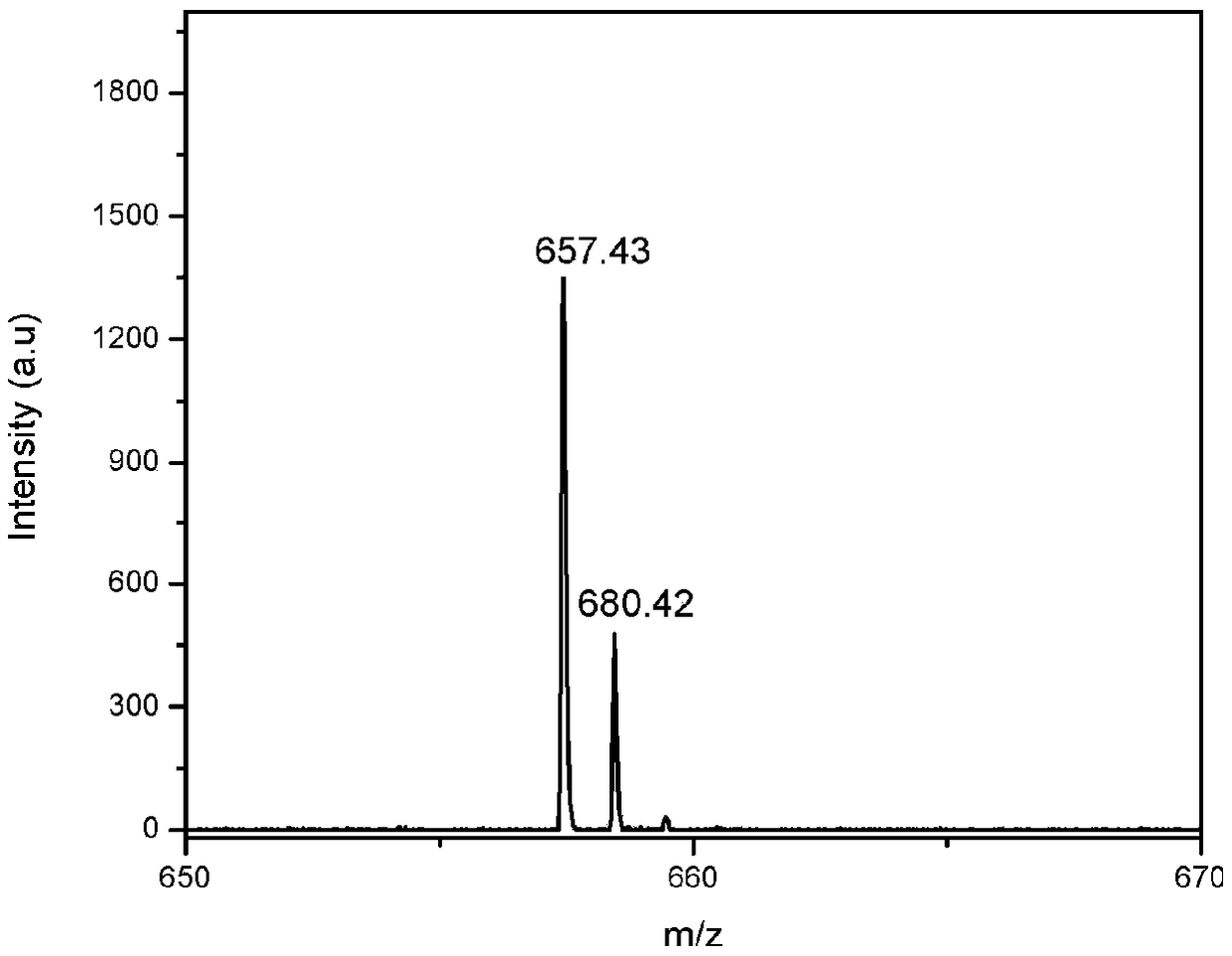
A side-chain liquid crystal polymer with aggregation-induced luminescent properties and its preparation method
A technology of aggregation-induced luminescence and side-chain liquid crystals, which is applied in the direction of liquid crystal materials, chemical instruments and methods, etc., to achieve the effect of simple synthesis, good liquid crystal performance, and obvious aggregation-induced luminescence effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Synthesis of cyano-stilbene derivatives
[0048] Add p-octadecyloxybenzaldehyde (10.0g, 26.7mmol), NaOH (2.14g, 53.4mmol) and 150ml of dehydrated alcohol successively in a 250ml single-necked bottle, then add p-hydroxyphenylacetonitrile (3.6g, 26.7 mmol), reflux at 85°C for 4 hours, then cool the reaction solution to room temperature, then pour it into a large amount of ice water for precipitation, filter with suction, wash the filter cake with dilute hydrochloric acid and deionized water successively, and collect the crude product under vacuum at 35°C After drying for 48 h, the dried crude product was recrystallized from ethyl acetate to obtain a pale yellow pure solid product.
[0049] 2. Synthesis of cyano-stilbene derivatives containing flexible spacers
[0050] Add 4-hydroxyl-4'-octadecyloxycyano-stilbene (4.5g, 9.2mmol), anhydrous potassium carbonate (3.8g, 27.6mmol) and the acetone of 150ml successively in a 250ml single-necked bottle, and then Add 1,6-dibro...
Embodiment 2
[0057] 1. Synthesis of cyano-stilbene derivatives
[0058] Same as Example 1
[0059] 2. Synthesis of cyano-stilbene derivatives containing flexible spacers
[0060] Add 4-hydroxy-4'-octadecyloxycyano-stilbene (10.0g, 20.4mmol), anhydrous potassium carbonate (8.5g, 61.2mmol) and 120ml of DMF successively in a 250ml single-necked bottle, and then Add 1,4-dibromobutane (8.8g, 40.8mmol) and react at 65°C for 24h under magnetic stirring in an oil bath, then slowly add the reaction solution to 1000ml of ice water for precipitation, suction filter, and vacuum dry at 35°C for 48h , the crude product was filtered with dichloromethane to remove insoluble solid impurities, and the filtrate was spin-dried to obtain a light blue solid powder.
[0061] 3. Synthesis of flexible spacer monomers
[0062] In a 250ml round bottom flask, add 4-bromobutoxy-4'-hexyloxycyano toluene (15.0g, 24.0mmol), then add potassium bicarbonate (7.2g, 71.2mmol), DMF120ml and Methacrylic acid (2.1g, 48.0mmol...
Embodiment 3
[0067] 1. Synthesis of cyano-stilbene derivatives
[0068] Same as Example 1
[0069] 2. Synthesis of cyano-stilbene derivatives containing flexible spacers
[0070] Add 4-hydroxyl-4'-octadecyloxycyano-stilbene (10.0g, 20.4mmol), anhydrous potassium carbonate (7.1g, 51.1mmol) and 120ml of acetone successively in a 250ml single-necked bottle, and then Add 1,8-dibromooctane (8.3g, 30.6mmol) and react at 65°C for 16h under magnetic stirring in an oil bath, then slowly add the reaction solution into 1000ml of ice water to precipitate, filter with suction, and dry in vacuum at 35°C for 48h , the crude product was filtered with dichloromethane to remove insoluble solid impurities, and the filtrate was spin-dried to obtain a light blue solid powder.
[0071] 3. Synthesis of flexible spacer monomers
[0072] In a 250ml round bottom flask, add 4-bromooctyloxy-4'-octadecyloxycyano-stilbene (20.0g, 29.4mmol), then add potassium bicarbonate (7.4g, 73.6mmol), Acetone 150ml and methacry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com