Folate-modified medicine-carrying hydroxylapatite and preparation method thereof
A technology of drug-loaded hydroxyapatite and hydroxyapatite, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as normal tissue damage and damage, and achieve High load capacity, reduced leakage rate, good chemical stability and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The present invention aims to provide a preparation method of folic acid-modified drug-loaded hydroxyapatite (FA@D@HAP), and a product prepared by the method. The preparation method includes: first preparing drug-loaded hydroxyapatite (D@HAP), and using a silane coupling agent or an amino polymer to carry out surface amination treatment, and using folic acid to aminate the surface The step of modifying the drug-loaded hydroxyapatite.
[0022] Unless otherwise specified, the symbols FA used in this manual represent folic acid, D represents the drug contained, DOX represents doxorubicin, HAP represents hydroxyapatite, D@HAP represents drug-loaded hydroxyapatite, FA@ D@HAP stands for folate-modified drug-loaded hydroxyapatite.
[0023] In the specific embodiment, in the method, drug-loaded hydroxyapatite is prepared by gas-liquid precipitation hydrothermal method: water-soluble calcium salt, phosphate and drug are prepared by reacting in an ethanol-water system at 100-120...
Embodiment 1
[0040] Example 1. Preparation of FA@DOX@HAP
[0041] 1.1 Synthesis of drug-loaded hydroxyapatite (DOX@HAP)
[0042] Adopt gas-liquid precipitation hydrothermal method to combine, weigh 30mg doxorubicin hydrochloride, 1.490g Ca(NO 3 ) 2 4H 2 O and 0.495g of (NH 4 ) 2 HPO 4 respectively dissolved in 2mL of ultrapure water, 30mL of absolute ethanol and 2mL of ultrapure water, and Ca(NO 3 ) 2 4H 2 The pH of the ethanol solution of O was adjusted to about 10.5. In this alkaline environment, slowly add the aqueous solution of doxorubicin hydrochloride dropwise while stirring, and after stabilizing for 10 minutes, continue to add dropwise (NH 4 ) 2 HPO 4 Aqueous solution, after the dropwise addition, continue to stir for 1 hour, then pour the turbid solution into the reaction kettle, conduct a hydrothermal reaction at 120°C for 24 hours, then centrifuge at a high speed, wash the product with ultrapure water and absolute ethanol several times, vacuum it at 60°C Dry for 12 ...
Embodiment 2
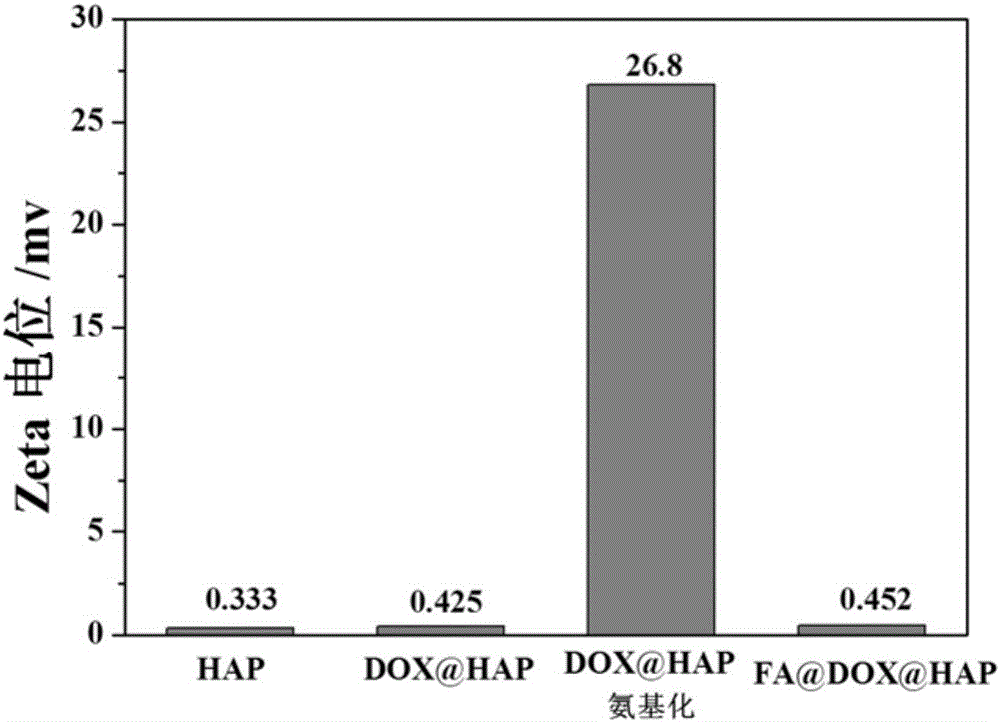
[0047] Example 2. Test of basic properties of drug-loaded hydroxyapatite modified by folic acid
[0048] 2.1 Size and morphology of folic acid-modified drug-loaded hydroxyapatite
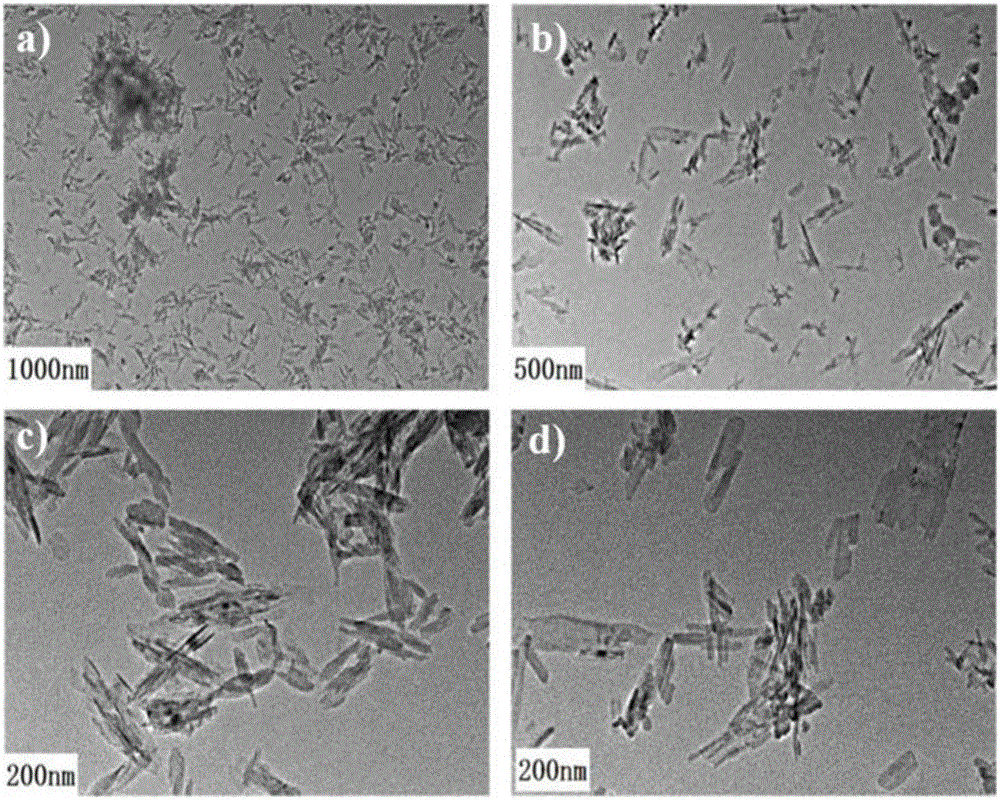
[0049] The morphology and size of the folic acid-modified drug-loaded hydroxyapatite obtained in Example 1 is determined by observation with a transmission electron microscope, and the test results are shown in figure 1 . It can be clearly seen from the transmission electron microscope pictures that the FA@DOX@HAP nanoparticles synthesized by co-precipitation and hydrothermal combination are rod-shaped, with an average length of 50-100nm and an average diameter of 5-20nm, and are not easy to aggregate. Good dispersion.
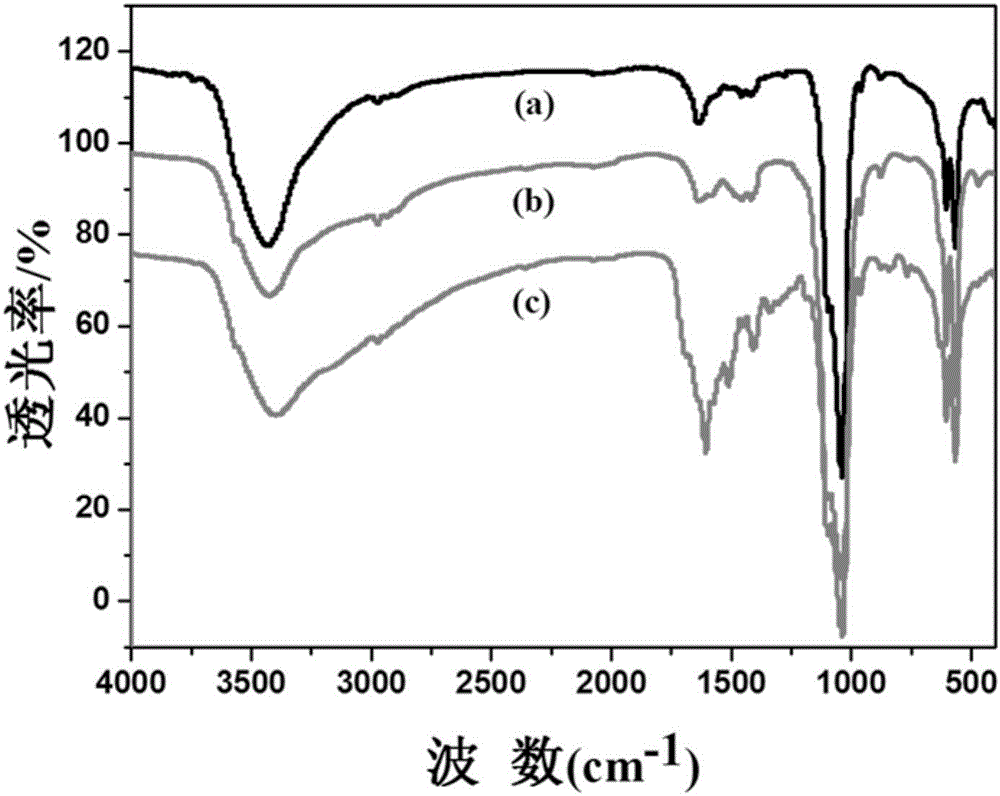
[0050] 2.2 Advanced Fourier Transform Infrared Spectroscopy
[0051] Take 1-2mg folic acid-modified drug-loaded hydroxyapatite sample, mix it with dry potassium bromide powder (A.R. grade) in an agate mortar and grind it into a fine powder, put it into a mold, and compress it in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com