Synthesis method of 3,4-diaminotoluene
A technology of diaminotoluene and a synthesis method, applied in the field of organic synthesis, can solve the problems of low content of finished products, brown appearance, unstable quality, etc., and achieve the effects of reduced raw material cost, high product purity, and fewer reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
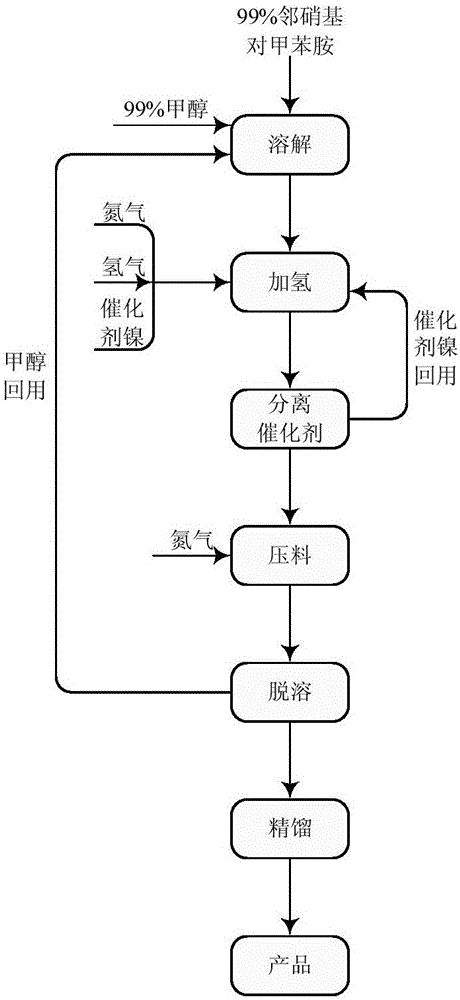
[0016] Add 300 grams of o-nitro-p-toluidine to a 1500 ml three-necked flask equipped with a reflux condenser, then add 450 grams of methanol, stir, and heat the material to 50-55 ° C for 30 minutes to dissolve. Add the above-mentioned methanol solution of o-nitro-p-toluidine to a 1-liter autoclave, then add 30 grams of Ni catalyst, and replace with nitrogen and hydrogen. Start stirring and heat up to 60°C, start hydrogenation, control hydrogenation pressure 1-3MPa, temperature 65-75°C, maintain for 1 hour after hydrogenation, exhaust hydrogen, discharge, remove methanol under normal pressure, and then under reduced pressure The water was removed, and finally 233.6 g of 3,4-diaminotoluene was obtained through rectification under reduced pressure, with a content of 99.5% and a yield of 97%.
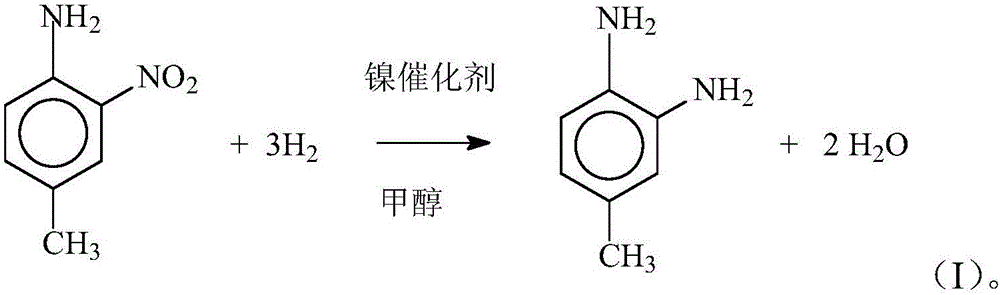
[0017] Concrete reaction steps are shown in formula (I):
[0018]
Embodiment 2
[0020] Add 300KG o-nitro-p-toluidine into a 1500L enamel reaction kettle equipped with a reflux condenser, then add methanol 450kg methanol, start stirring, and raise the temperature of the material to 50-50 to dissolve for 30 minutes. The above-mentioned methanol solution of o-nitro-p-toluidine was poured into a 1000-liter autoclave with a stainless steel pump, and then 30 kg of Ni catalyst was added, and replaced with nitrogen and hydrogen. Turn on stirring and heat up to 60°C, start hydrogenation, control hydrogenation pressure 1-3MPa, temperature 65-75°C, keep hydrogenation for 1 hour, exhaust hydrogen, discharge, methanol is removed under normal pressure, and then under reduced pressure The water was removed, and finally 233kg of 3,4-diaminotoluene was obtained through rectification under reduced pressure, with a content of 99.6% and a yield of 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com