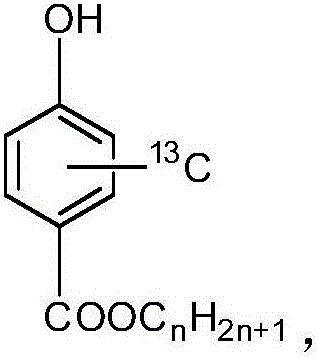
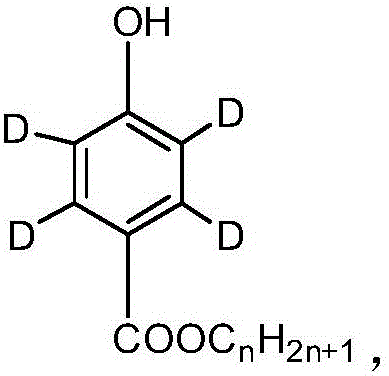
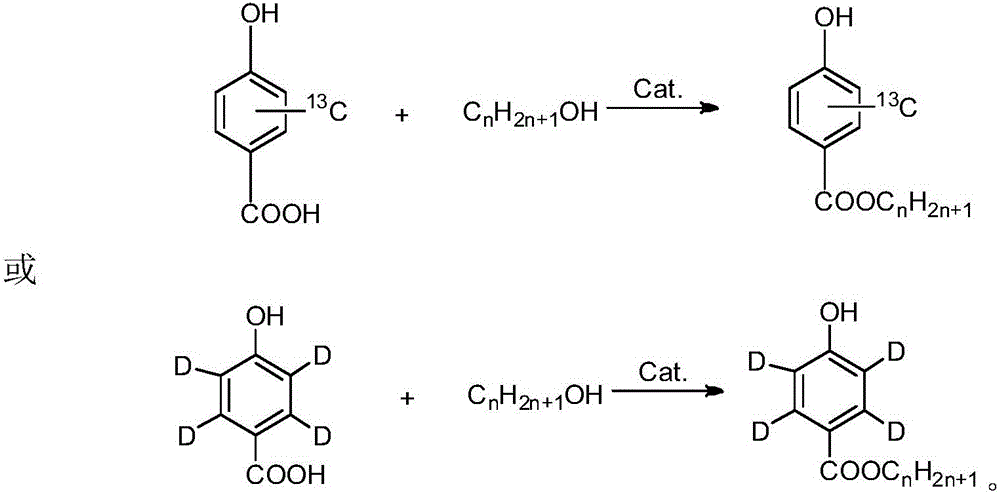
Synthesis method of paraben compounds marked by stable isotope 13C or D
A p-hydroxybenzoic acid and stable isotope technology, applied in the field of stable isotope labeling compounds, can solve the problems of large corrosion equipment and waste liquid pollution, large amount of sulfuric acid catalyst, and reduced isotope abundance, etc., to achieve low corrosion and waste liquid pollution, Good economy and practical application value, the effect of reducing product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Stable isotope labelled methyl parahydroxybenzoate- 13 C 6 The synthesis method of the method, the specific synthesis process of the method includes the following steps:
[0029] In a three-necked flask equipped with magnetic stirring, thermometer and reflux condenser, add 7.2g of p-hydroxybenzoic acid- 13 C 6 (0.05mol), 8.1mL methanol, 4.13g p-toluenesulfonic acid, heated to 60°C, kept the reaction for 9 hours under stirring; after the reaction, cooled to room temperature, filtered with suction, and adjusted the filtrate to pH=7~8, suction filtration, vacuum drying oven at 50℃ to constant weight, to obtain 5.17g white powder, with p-hydroxybenzoic acid- 13 C 6 The calculated yield is 65.4%, and methyl p-hydroxybenzoate- 13 C 6 The chemical purity is 99.11%, and the isotopic abundance is 99.21atom% 13 C.
Embodiment 2
[0031] Stable isotope labeled ethyl p-hydroxybenzoate- 13 C 6 The synthesis method of the method, the specific synthesis process of the method includes the following steps:
[0032] In a three-necked flask equipped with magnetic stirring, thermometer and reflux condenser, add 7.2g of p-hydroxybenzoic acid- 13 C 6 (0.05mol), 17.5mL of ethanol, 4.13g of p-toluenesulfonic acid, heated to 90°C, kept the reaction for 8 hours under stirring; after the reaction, cooled to room temperature, filtered with suction, and adjusted the filtrate to pH=7~8, suction filtration, vacuum drying oven at 50℃ to constant weight, obtain 5.79g white powder, and p-hydroxybenzoic acid- 13 C 6 The calculated yield is 67.3%, ethyl p-hydroxybenzoate- 13 C 6 The chemical purity is 99.05%, and the isotopic abundance is 99.13atom% 13 C.
Embodiment 3
[0034] Stable Isotope Labeled Propylparaben- 13 C 6 The synthesis method of the method, the specific synthesis process of the method includes the following steps:
[0035] In a three-necked flask equipped with magnetic stirring, thermometer and reflux condenser, add 7.2g of p-hydroxybenzoic acid- 13 C 6 (0.05mol), 23.4mL n-propanol, 4.13g p-toluenesulfonic acid, warm up to 65°C, keep the reaction for 12 hours while stirring; after the reaction is over, cool to room temperature, filter with suction, and use 10% NaOH solution to filter the filtrate Adjust to pH=7-8, filter by suction, and dry to constant weight at 50°C in a vacuum drying oven to obtain 6.53g of white powder with p-hydroxybenzoic acid- 13 C 6 The calculated yield is 70.2%, propyl p-hydroxybenzoate- 13 C 6 The chemical purity is 99.13%, and the isotopic abundance is 99.15atom% 13 C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com