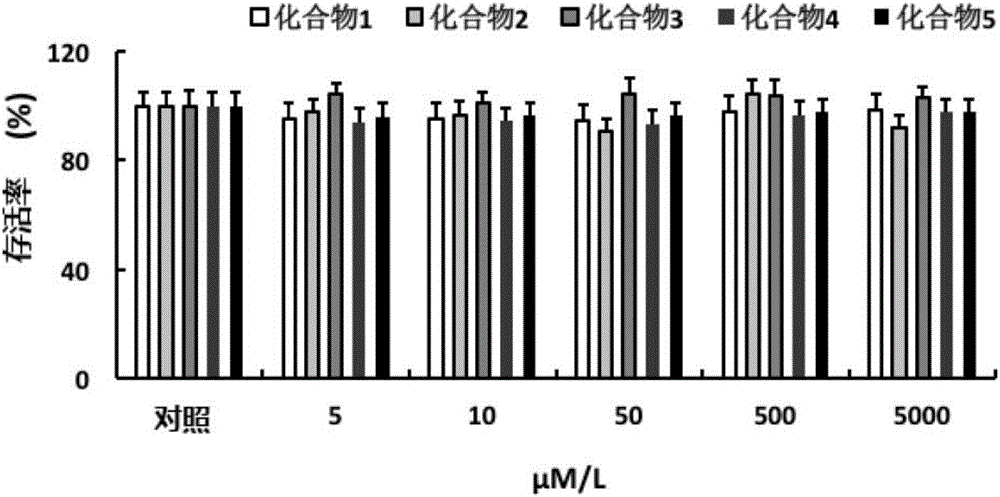
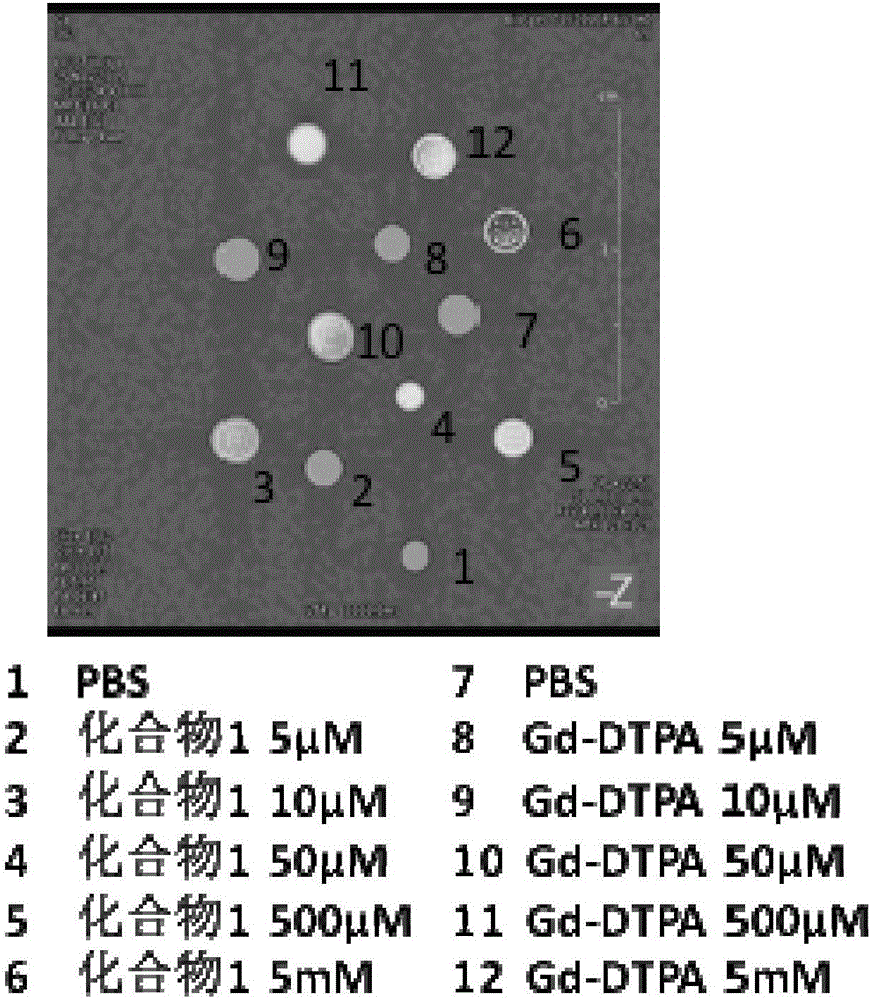
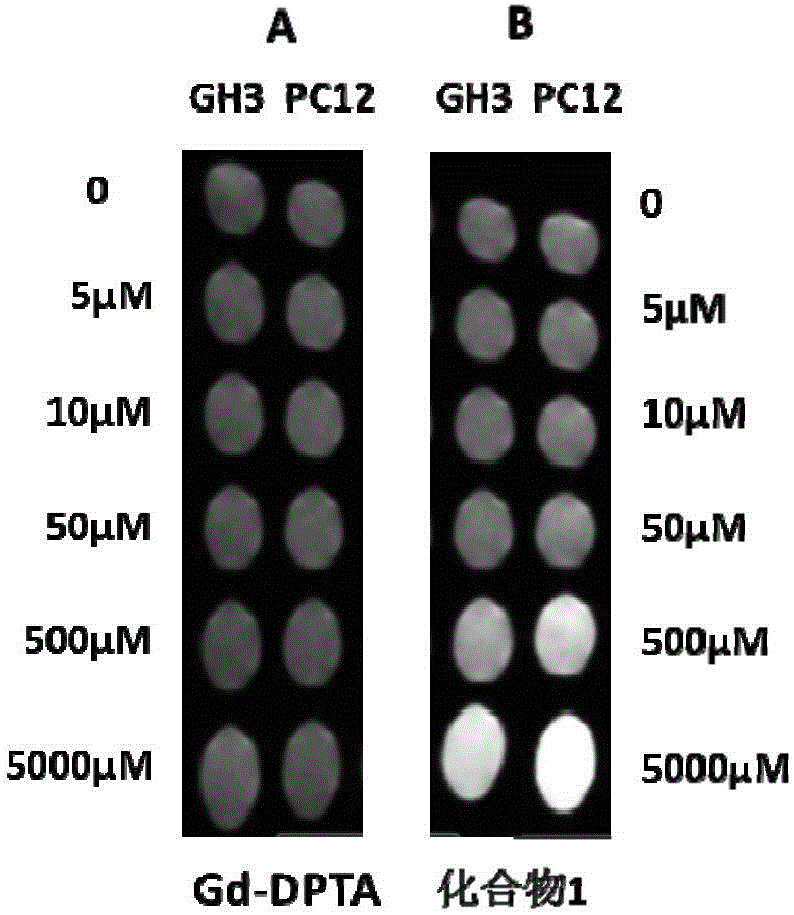
Pyrrolidine compounds, salts thereof, applications of compounds or salts thereof in nuclear magnetic probes and medicines, reagent, and medicine
The technology of a compound, benzamidomethylpyrrolidine, is applied in the fields of pyrrolidine compounds, salts, nuclear magnetic probes and pharmaceutical applications, reagents, and drugs, and can solve problems such as the inability to accurately detect the distribution density of dopamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0089] Preparation example 1. Synthesis of O-benzyl-3-butyn-1-ol
[0090]
[0091] Dissolve and suspend 1.76g of 60% NaH (reagent concentration: 60%) in 40ml of anhydrous DMF (dimethylformamide), add 2.8g of 3-butyn-1-ol under the protection of argon in the ice water external bath , continue to react for 30 minutes after no gas is produced, drop 5.24ml of benzyl bromide, remove the ice-water bath after dropping, and react at room temperature for 12 hours, after stopping the reaction, add 200ml of 1mol / L hydrochloric acid aqueous solution, add EtOAc (ethyl acetate) 100ml to extract 3 times, the organic layer was washed 3 times with 1mol / L hydrochloric acid aqueous solution and saturated NaCl solution, and then washed with Na 2 SO 4 After drying and filtering, the filtrate was evaporated to dryness, and the fraction at 80°C under a pressure of 4 mmHg was collected by vacuum distillation to obtain 3.407 g of a colorless oily product with a yield of 53.2%.
[0092] Product an...
preparation example 2
[0093] Preparation example 2. Synthesis of O-benzyl-4-pentyn-1-ol
[0094]
[0095] Dissolve and suspend 1.76g of 60% NaH in 40ml of anhydrous DMF, add 3.36g of 3-pentyn-1-ol under the protection of argon in an external bath of ice water, continue to react for 30 minutes after no gas is generated, and then add 5.24ml of bromine Benzyl, remove the ice-water bath after dropping, react at room temperature for 12 hours, add 1mol / L hydrochloric acid aqueous solution 200ml after stopping the reaction, add EtOAc 100ml to extract 3 times, the organic layer is washed 3 times with 1mol / L hydrochloric acid aqueous solution and saturated NaCl solution, and NaCl solution 2 SO 4 After drying and filtering, the filtrate was evaporated to dryness, and the fraction at 95°C under a pressure of 4 mmHg was collected by vacuum distillation to obtain 3.967 g of a colorless oily product with a yield of 57%.
[0096] Product analysis: 1 H NMR (400MHz, CDCl 3 )δ7.31(m,5H),4.47(s,2H),3.43(t,J=4.7...
preparation example 3
[0097] Preparation example 3. Synthesis of O-benzyl-5-hexyn-1-ol
[0098]
[0099] Dissolve and suspend 0.876g 60% NaH in 20ml of anhydrous DMF, add 1.95g of 3-hexyn-1-ol under the protection of argon in an external bath of ice water, continue to react for 30 minutes after no gas is generated, and then add 2.6ml of bromine Benzyl, remove the ice-water bath after dropping, and react at room temperature for 12 hours. After stopping the reaction, add 100ml of 1mol / L hydrochloric acid aqueous solution, add 50ml of EtOAc to extract 3 times, and the organic layer is washed 3 times with 1mol / L hydrochloric acid aqueous solution and saturated NaCl solution. 2 SO 4 After drying and filtering, the filtrate was evaporated to dryness, and the fraction at 110° C. under a pressure of 4 mmHg was collected by vacuum distillation to obtain 3.016 g of a colorless oily product with a yield of 80.6%.
[0100] Product analysis: 1 H NMR (400MHz, CDCl 3 )δ7.33(m,5H),4.42(s,2H),3.54(t,J=4.6Hz,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com