Method for preparing nitric acid, calcium fluoride and potassium fluosilicate through using waste fluorine-containing nitric acid
A technology of potassium fluorosilicate and calcium fluoride, which is applied in the direction of fluorosilicic acid, nitric acid, silicon halide compounds, etc., can solve the problems of waste of fluorine and nitric acid resources, and achieve the effects of energy saving, easy control of process conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
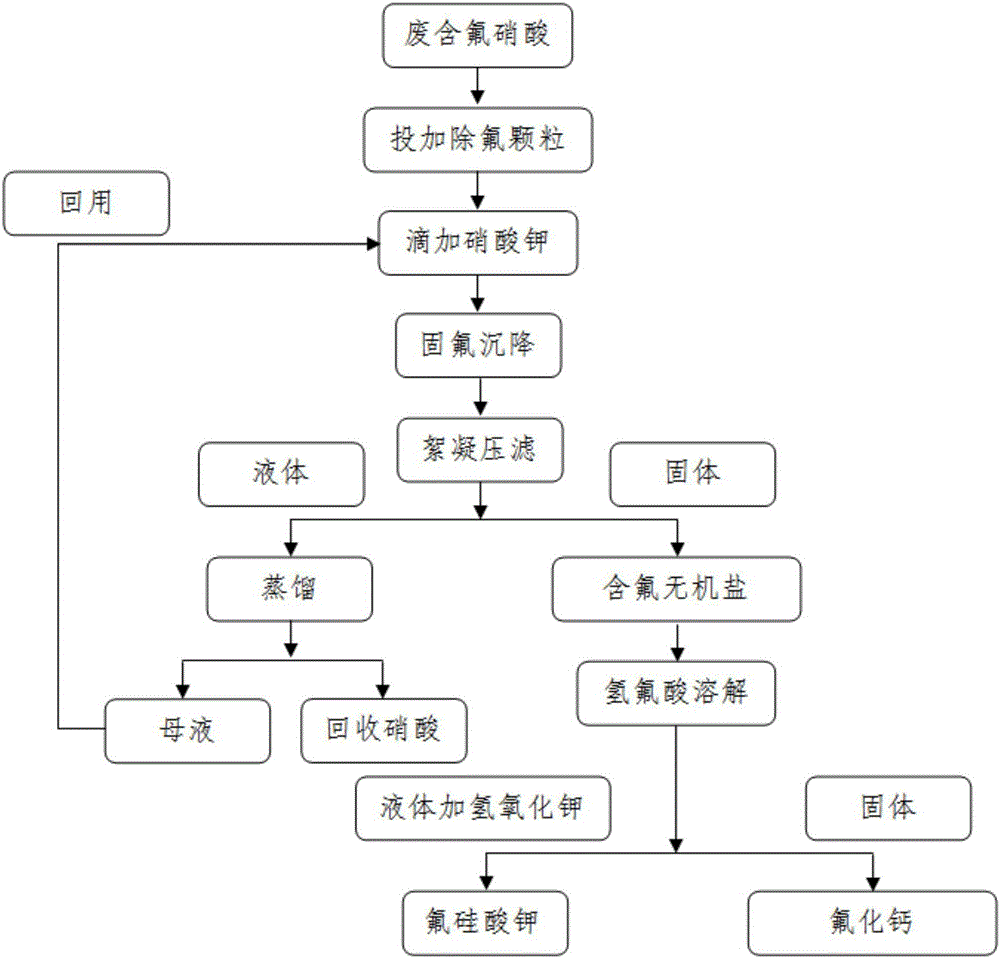
[0022] Take 200ml of waste fluorine-containing nitric acid from the production line of a solar cell manufacturer, add 5g of fluorine-removing granular silicon dioxide, 3g of kaolin, and 5g of garnet to the fluorine-containing nitric acid and stir for 30 minutes. After stirring, let it stand for 15 minutes, and then add 30ml of 70wt% potassium nitrate solution was used to fix fluorine (the drop rate was 0.3ml per minute), and the whole process was kept at 85°C. The obtained solid-liquid mixture is put into 1ml of flocculant (0.5wt% polyacrylamide solution) and fully stirred, flocculated and settled, and after standing for 50 minutes, the solid-liquid separation is carried out by pressure filtration through a PD membrane, and the liquid is reduced at 0.05Mpa at 130°C. Distill under high pressure to obtain pure nitric acid, and collect the distilled mother liquor for reuse. Centrifuge the separated solid and add 40ml of hydrofluoric acid to dissolve it. The obtained solid is calc...
Embodiment 2
[0024] Take 150ml of waste fluorine-containing nitric acid from the production line of a certain solar cell manufacturer, add 6g of fluorine-removing granular silicon dioxide and 4g of kaolin to the fluorine-containing nitric acid and stir for 30 minutes. After stirring, let stand for 20 minutes, then add 65wt% potassium nitrate dropwise The solution is 20ml of fluorine fixation (the drop rate is 1ml per minute), and the whole process is kept at 80°C. Put the obtained solid-liquid mixture into 0.5ml of flocculant (0.1wt% polyacrylamide solution) and stir fully, flocculate and settle, let stand for 30 minutes, press filter and separate solid-liquid by centrifugation, the liquid is at 0.07Mpa, 120°C Carry out vacuum distillation to obtain pure nitric acid, and the mother liquor of distillation is collected for reuse. Centrifuge the separated solid and add 45ml of hydrofluoric acid to dissolve it. The obtained solid is calcium fluoride. Add 25g of potassium hydroxide to the liqui...
Embodiment 3
[0026] Take 400ml of waste fluorine-containing nitric acid from the production line of a certain solar cell manufacturer, add fluorine-removing particle silicon dioxide 11g and garnet 9g to the fluorine-containing nitric acid and stir for 60 minutes. After stirring, let stand for 30 minutes, then add 75wt% nitric acid dropwise Potassium solution 57ml fixes fluorine (the drop rate is 1.5ml per minute), and the whole process is kept at 80°C. Put the obtained solid-liquid mixture into 1.5ml of flocculant (1wt% polyacrylamide solution) and fully stir, flocculate and settle, let stand for 40 minutes, press filter and separate the solid and liquid by centrifugation, the liquid is carried out at 0.09Mpa, 110°C Distill under reduced pressure to obtain pure nitric acid, and collect the distilled mother liquor for reuse. Centrifuge the separated solid and add 50ml of hydrofluoric acid to dissolve it. The obtained solid is calcium fluoride. Add 21g of potassium hydroxide to the liquid to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com