Hydrobromide of benzodiazepine derivative and multiple crystal forms thereof, and preparation method for multiple crystal forms
A technology of hydrobromide and hydrobromic acid, applied in the field of hydrobromide and its polymorphs and their preparation, can solve problems such as hidden dangers of drug safety, ethanol residue, etc., and achieves low solvent residue, low cost, potential Low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of the hydrobromide I crystal form of formula (I) compound
[0040] Accurately weigh 44 mg (0.10 mmol) of the compound of formula (I) in a 10 mL single-necked bottle, add 0.4 mL of ethyl acetate and stir to make it all dissolve, the reaction temperature is down to 4 ° C, and then 1.1 mL of hydrobromic acid methanol solution (1mol / L, 0.11mmol) was added dropwise to the ethyl acetate solution of the compound of formula (I), stirred and crystallized, filtered with suction, rinsed with ethyl acetate, and dried under reduced pressure at 30°C to obtain the hydrobromide of the compound of formula (I) , white solid 42mg, yield 81%.
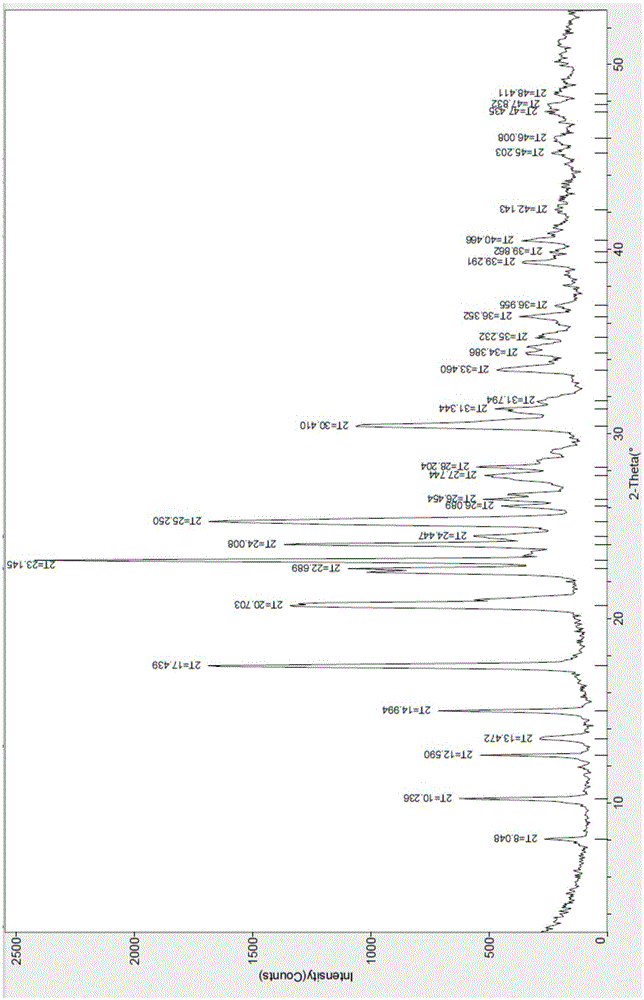
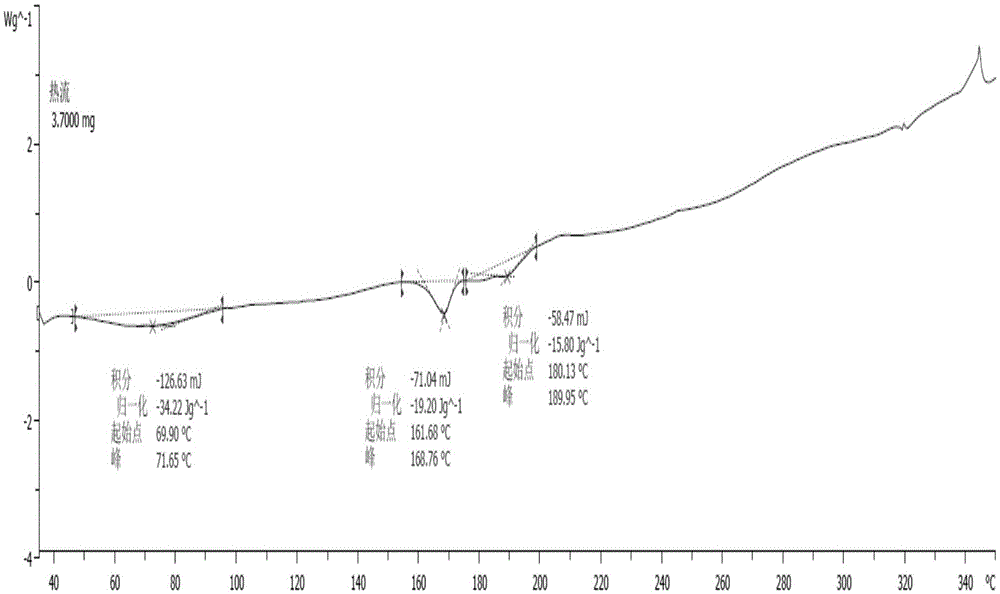
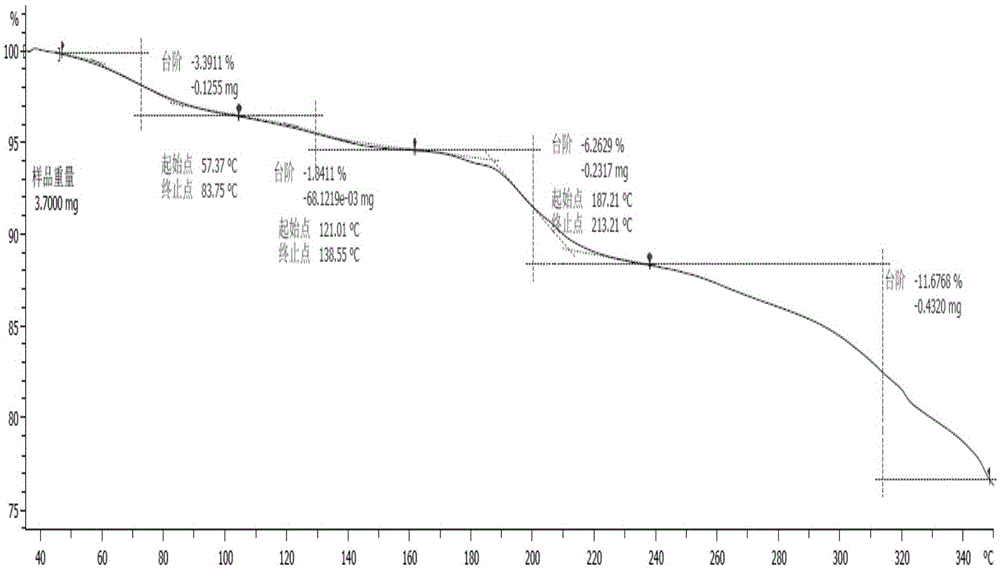
[0041] The X-ray diffraction spectrum figure of this crystal is shown in the attached figure 1 , with characteristic peaks at about 8.05, 10.24, 12.59, 13.47, 14.99, 17.44, 20.70, 22.69, 23.15, 24.01, 24.45, 25.25, 26.09, 26.45, 27.74, 28.20, 30.41, 31.34, 33.46, 36.395, 40.2 , see the attached DSC spectrum figure 2...
Embodiment 2
[0042] Embodiment 2: the preparation of the hydrobromide I crystal form of formula (I) compound
[0043] Accurately weigh 44 mg (0.10 mmol) of the compound of formula (I) in a 10 mL single-necked bottle, add 0.4 mL of acetone and stir to make it completely dissolved, and the reaction temperature is lowered to 4 ° C, then 1.1 mL of methanolic hydrobromic acid (1 mol / L , 0.11mmol) was added dropwise to the acetone solution of the compound of formula (I), stirred and crystallized, filtered by suction, washed with acetone, and dried under reduced pressure at 30°C to obtain the hydrobromide of the compound of formula (I), 37mg of white solid, yield 71%.
[0044] The X-ray diffraction spectrogram and the DSC spectrogram of the crystalline sample are studied and compared, and it is determined that the product is the hydrobromide I crystal form of the compound of formula (I).
Embodiment 3
[0045] Embodiment 3: the preparation of the hydrobromide II crystal form of formula (I) compound
[0046] Accurately weigh 22.26 mg of the compound of formula (I) in a 1 mL centrifuge tube, add 100 μL of acetone and stir to make it completely dissolved, then dissolve 10 mg of 47% aqueous hydrobromic acid in 75 μL of acetone, and add dropwise to the compound of formula (I) in acetone solution, stirred and crystallized, centrifuged, and dried under reduced pressure at 30° C. to obtain the hydrobromide salt of the compound of formula (I), 20 mg of white solid, with a yield of 76%.
[0047] The X-ray diffraction spectrum figure of this crystal is shown in the attached Figure 4 , at about 7.14, 8.78, 13.69, 14.34, 15.43, 17.26, 17.89, 18.22, 18.77, 19.69, 20.07, 20.49, 22.30, 22.89, 23.75, 25.03, 25.51, 26.51, 27.47, 230.70, 28.20.83 , 33.23, 33.92, 36.03, and 38.38 have characteristic peaks, and the DSC spectrum is attached Figure 5 , there are characteristic absorption peaks ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com