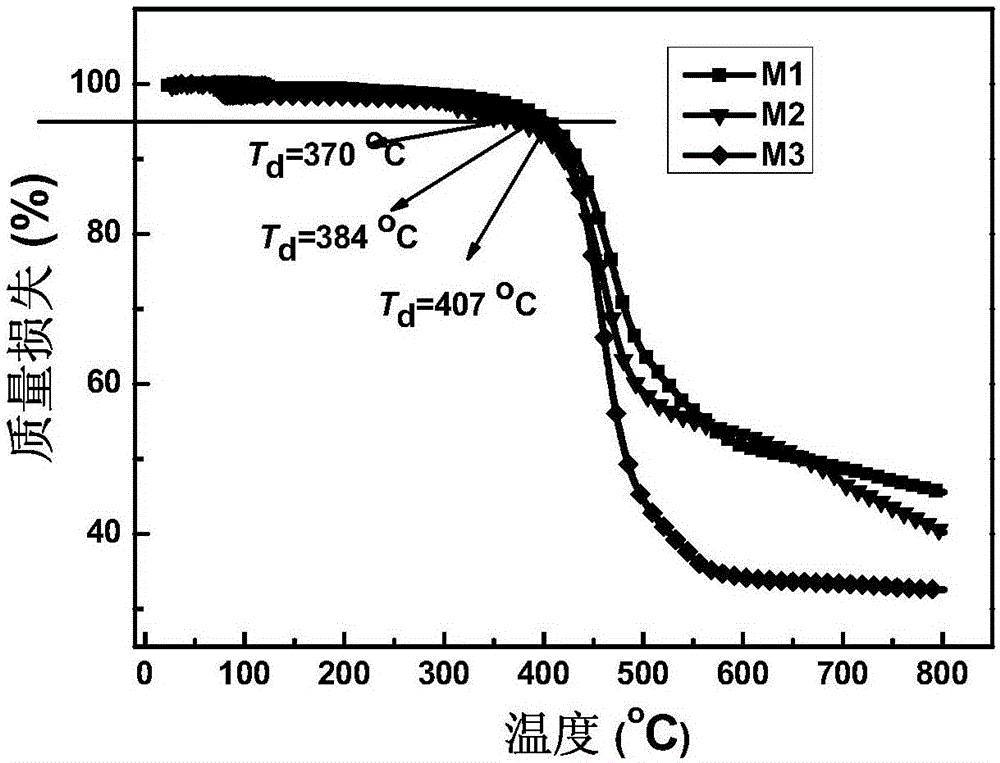
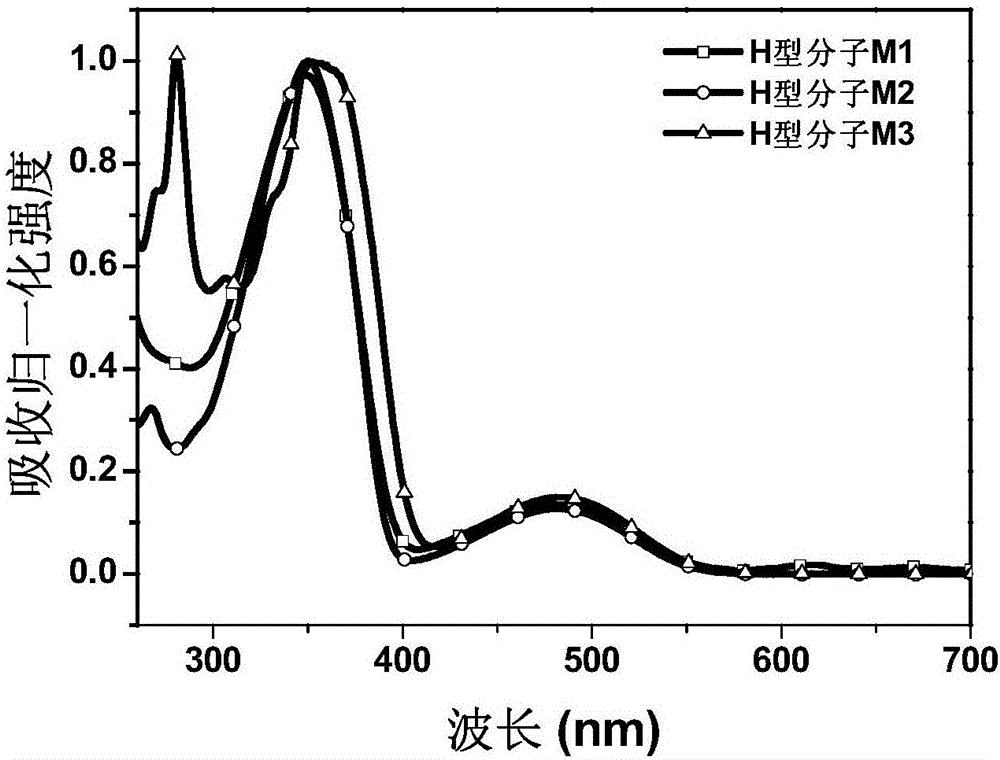
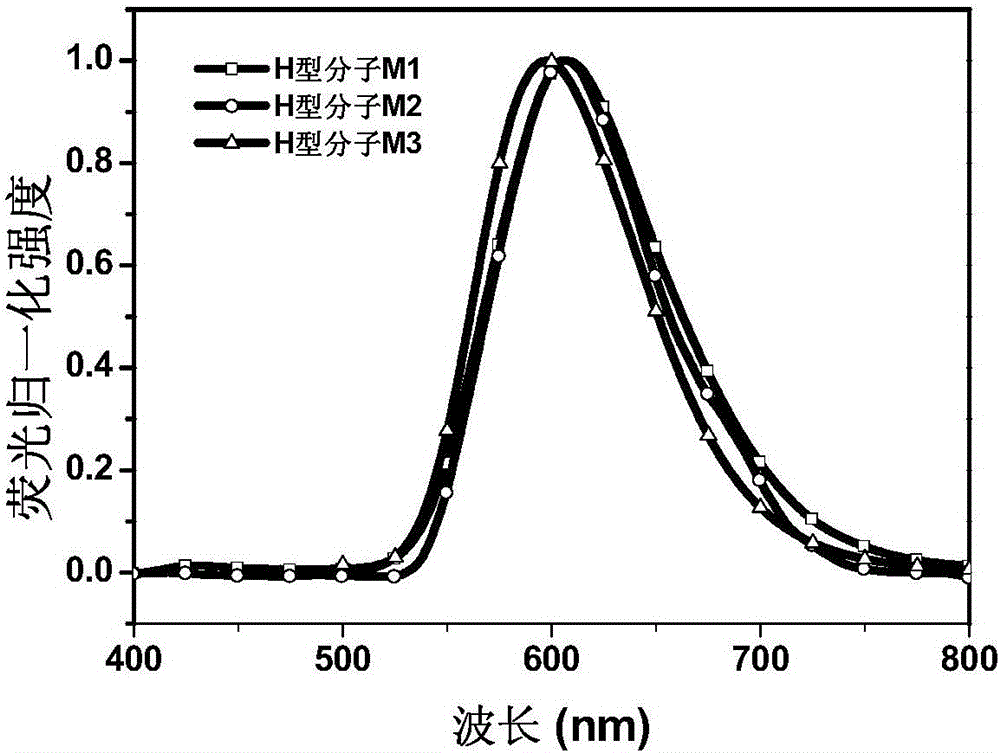
Fluorenyl donor-acceptor H type molecular material with high fluorescence quantum efficiency and preparation method and applications thereof
A fluorescent quantum and molecular material technology, applied in the field of organic semiconductor materials, achieves the effects of high fluorescent quantum efficiency, multiple options, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] High fluorescence quantum efficiency fluorene-based donor-acceptor H-type molecular material M1, wherein: Ar 1 , Ar 2 , Ar 3 , Ar 4 is phenyl, Ar 5 , Ar 6 is p-bromooctoxybenzene, Ar 7 , Ar 8 , Ar 9 , Ar 10 is fluorenyl tertiary alcohol, Ar 11 It is 4,7-bis(4-octylthiophen-2-yl)benzo[c][1,2,5]thiadiazole, and the preparation method includes the following steps:
[0053] (1) 3-Octylthiophene (5.0g, 25.5mmol) was added to 80mL of anhydrous THF, cooled to -78°C, n-butyllithium (2.4M in hexanes, 11.7ml, 28.0mmol) was slowly added dropwise, the dropwise addition was completed , stirred at -78 ° C for 1 hour, raised to room temperature and reacted for 1 hour, the reaction system was cooled to -78 ° C, and the THF solution of 2M trimethyltin chloride (9.1 g, 28.0 mmol) was added to the reaction system through a syringe for 1 After hours, the reaction was warmed to room temperature and stirred overnight. 20 mL of KF aqueous solution was added to the reactant to quenc...
Embodiment 2
[0059] High fluorescence quantum efficiency fluorene-based donor-acceptor H-type molecular material M2, wherein: Ar1 , Ar 2 , Ar 3 , Ar 4 is phenyl, Ar 5 , Ar 6 is p-bromooctoxybenzene, Ar 7 , Ar 8 , Ar 9 , Ar 10 for fluorene, Ar 11 It is 4,7-bis(4-octylthiophen-2-yl)benzo[c][1,2,5]thiadiazole, and the preparation method is the same as that in Example 1, except that the fluorenyl group in Example 1 The boronate ester 5a of tertiary alcohol was replaced with boronate ester 5b of fluorene, and finally the product M2 (40%) was obtained as a yellow oil. 1 H NMR (400MHz, CDCl 3 ): δ(ppm): 8.05(s, 2H), 7.88(m, 8H), 7.70(m, 12H), 7.58(m, 10H), 7.49(d, J=8.8Hz, 8H), 7.31(m ,12H),6.83(d,J=8.8Hz,4H),3.90(t,J=6.4Hz,4H),2.26(m,4H),1.97(m,16H),1.73(m,4H),1.18 (br,124H),0.88(m,34H).0.65(m,12H). 13 C NMR (101MHz, CDCl 3 )δ158.43,152.60,152.23,151.42,151.00,143.80,141.74,141.27,140.73,140.45,140.05,138.50,136.58,135.18,131.56,128.56,127.16,126.98,126.75,126.09,125.47,125.38,124.8...
Embodiment 3
[0061] High fluorescence quantum efficiency fluorene-based donor-acceptor H-type molecular material M3, wherein: Ar 1 , Ar 2 , Ar 3 , Ar 4 is phenyl, Ar 5 , Ar 6 is p-bromooctoxybenzene, Ar 7 , Ar 8 , Ar 9 , Ar 10 for pyrene, Ar 11 It is 4,7-bis(4-octylthiophen-2-yl)benzo[c][1,2,5]thiadiazole, and the preparation method includes the following steps:
[0062] (1) Compound 3 (0.5 g, 0.92 mmol), pyrene boronate 6 (0.36 g, 1.1 mmol), Pd (PPh 3 ) 4 (53mg, 0.045mmol) was added to a 150ml round bottom flask, K 2 CO 3 (10ml, 2M) and toluene (15ml) were separately purged with nitrogen for 30min before use to remove oxygen from the solution. The above solutions were poured into round-bottomed flasks, respectively, and the solution was heated to 100° C. and refluxed for 48 hours. The solution was cooled to room temperature, poured into 80 mL of deionized water, extracted with dichloromethane, the combined organic layers were washed with water and saturated brine, then dried...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com