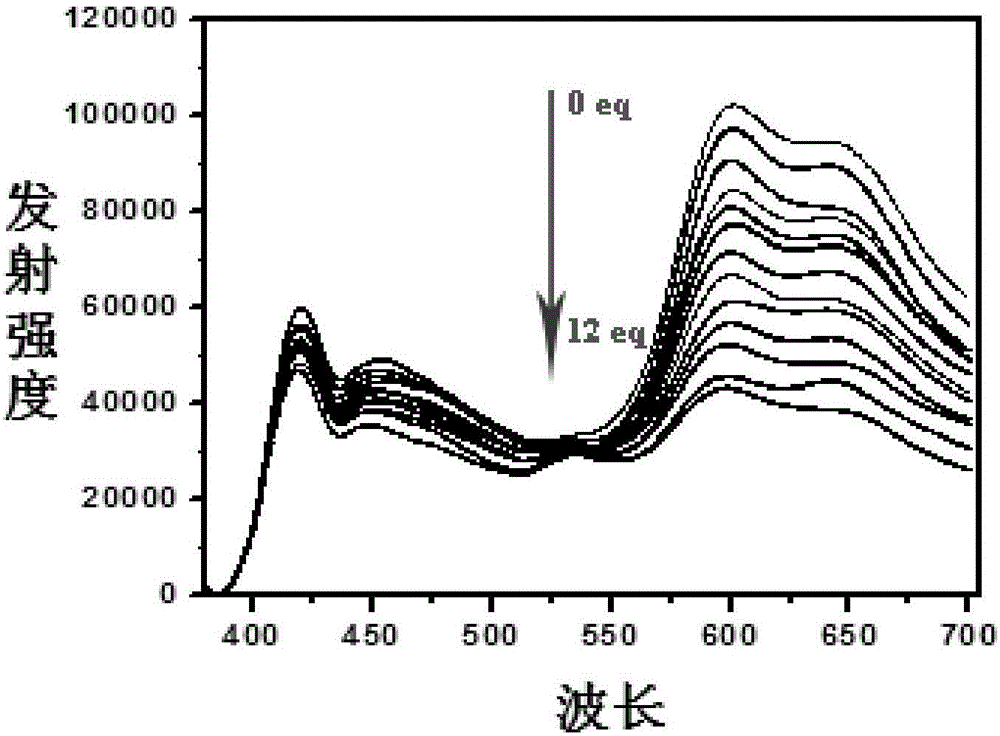
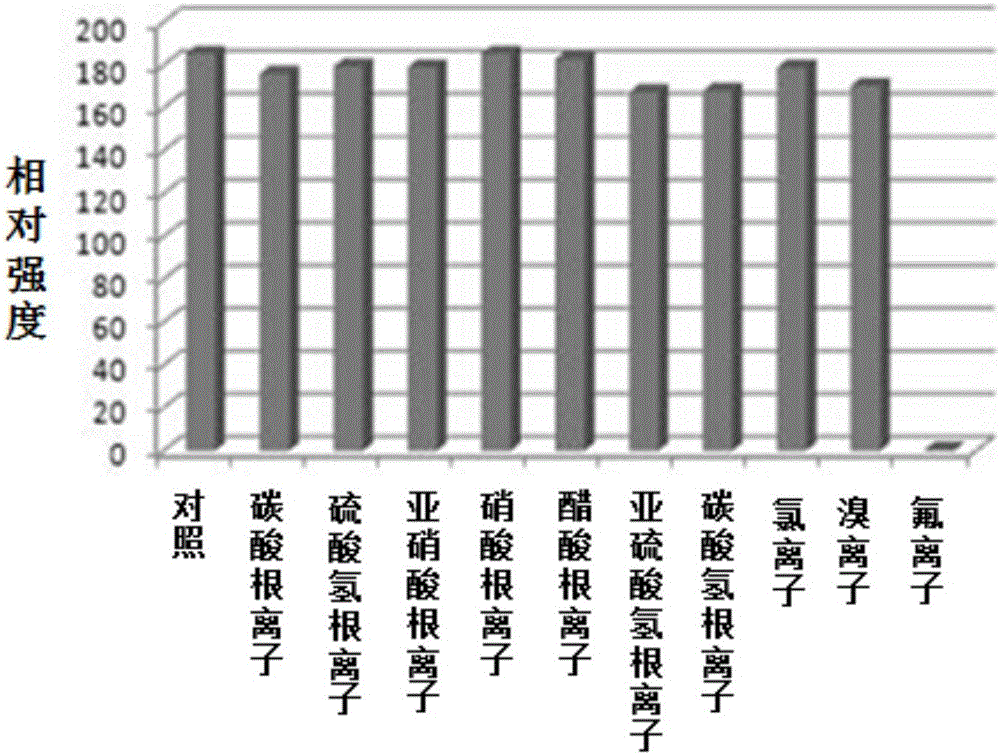
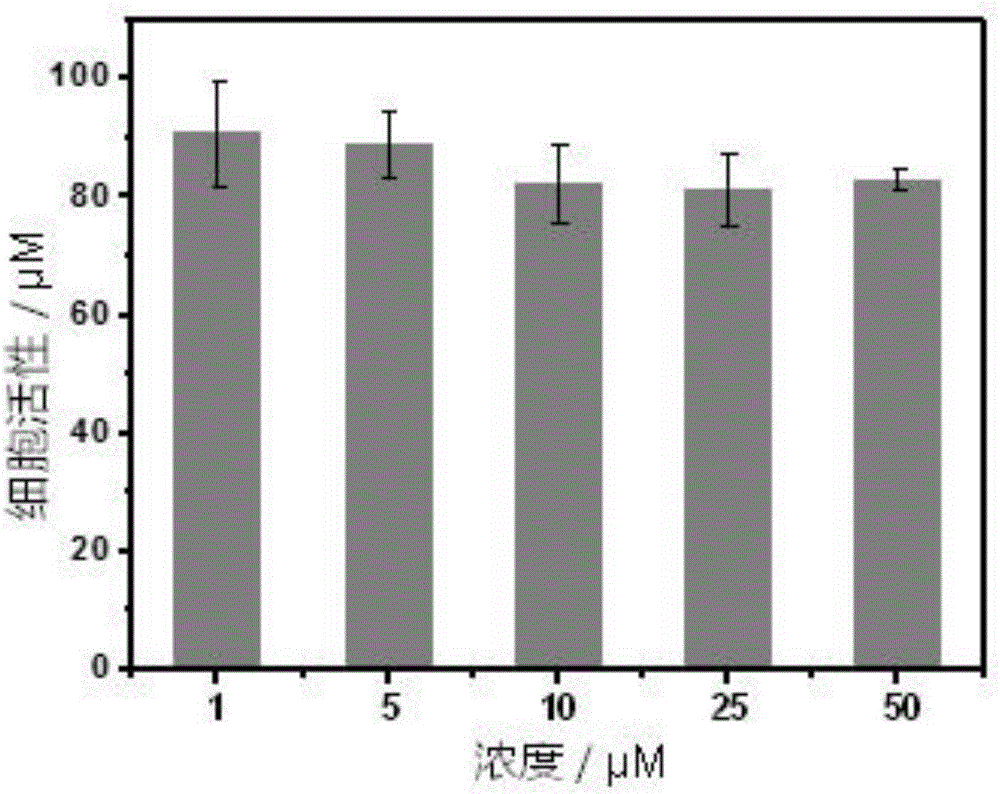
Ratio fluorescent fluorine ion probe, and preparation and applications thereof
A ratiometric fluorescence, fluoride ion technology, applied in the field of chemical/biosensors, can solve the problems of reducing detection sensitivity and signal-to-noise ratio, and achieve the effects of high selective detection, high sensitivity and improved accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Probe Ir1, namely Ligand is The preparation and application test of the probe material:
[0047] The preparation method of probe Ir1 is shown in the following equation and steps:
[0048]
[0049] (1) Preparation of Compound 3: Add 2,7-dibromo-fluorene (10.0g, 60.2mmol), finely powdered potassium hydroxide (1.69g, 30.1mmol) and 1-octanol (40g , 0.31mol), stirred and heated to 190°C for 19h. The reaction mixture was cooled to room temperature, and excess 1-octanol was distilled off under reduced pressure. The crude product was passed through a dry silica gel column with n-hexane as eluent to obtain 2,7-dibromo-9-octyl-fluorene (86%). 1 H NMR (400MHz, CDCl 3 ): δ=7.62(d, J=1.7Hz, 2H), 7.56(d, J=8.1Hz, 2H), 7.49(dd, J=1.7Hz and 8.1Hz, 2H), 3.95(t, J=5.8 Hz, 1H), 1.90-2.04(m, 2H, CH 2 ), 1.06-1.35 (m, 12H, 6×CH 2 ), 0.87(t, J=6.7Hz, 3H, CH 3 ); 13 C NMR (100MHz CDCl 3 ,): δ = 149.2, 139.0, 130.1, 127.6, 121.1, 121.0, 47.4, 32.6, 31.7, 29.7, 29.2, ...
Embodiment 2
[0066] Embodiment 2: probe Ir2, promptly when Ligand is The preparation and application test of the probe material:
[0067] The preparation method of probe Ir2 is shown in following equation and steps:
[0068] (1) Preparation of Compound 3: Add 2,7-dibromo-fluorene (10.0g, 60.2mmol), finely powdered potassium hydroxide (1.69g, 30.1mmol) and 1-octanol (40g , 0.31mol), stirred and heated to 150-220 ° C overnight. The reaction mixture was cooled to room temperature, and excess 1-octanol was distilled off under reduced pressure. The crude product was passed through a dry silica gel column with n-hexane as eluent to obtain 2,7-dibromo-9-octyl-fluorene (86%).
[0069] (2) Preparation of Compound 4: Add potassium hydroxide 51mmol and 2-pyridylbenzimidazole 10.2mmol in the reaction flask, and add 1-butyl-3-methylimidazolium hexafluorophosphate (ionic liquid) 20ml . After the mixture was pre-stirred for 5 min, 8 ml of 1,6-dibromohexane was added, and the reaction was stirre...
Embodiment 3
[0086] Embodiment 3: probe Ir2, promptly when Ligand is The preparation and application test of the probe material:
[0087] The preparation method of probe Ir3 is shown in following equation and steps:
[0088] The preparation process of compounds 3, 4 and 5 was consistent with that in Example 2.
[0089]
[0090] The preparation of compound 6c: Weigh compound 5 (0.71g, 1mmol), phenylboronic acid (0.28g, 2.3mmol) and tetrakis (triphenylphosphine) palladium (0.05g, 0.04mmol) join in two-necked flask, and to The reaction device is protected from light, and then vacuum-filled with nitrogen-vacuumized on the double-row tube, and the cycle is three times, and finally the reaction system is protected with nitrogen. 2M aqueous sodium carbonate solution (2.5 mL), toluene (5.0 mL) and ethanol (2.5 mL) were deoxygenated by bubbling nitrogen gas respectively, and injected into the reaction system with a syringe. Raise the temperature to 30-110°C. Then, the reaction system was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com