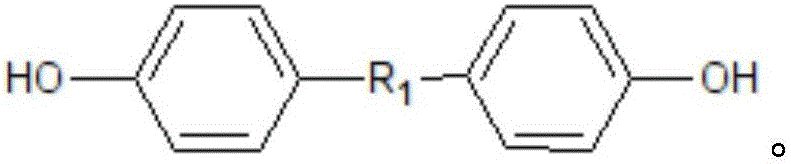Benzoxazine intermediate, and preparation method and application thereof
A technology of benzoxazine and intermediates, which is applied in the field of resin preparation, can solve the problems of short storage time, difficulty in realizing industrial production, and limited application, and achieve the goals of shortening gelation time, realizing industrial production, and extending storage time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
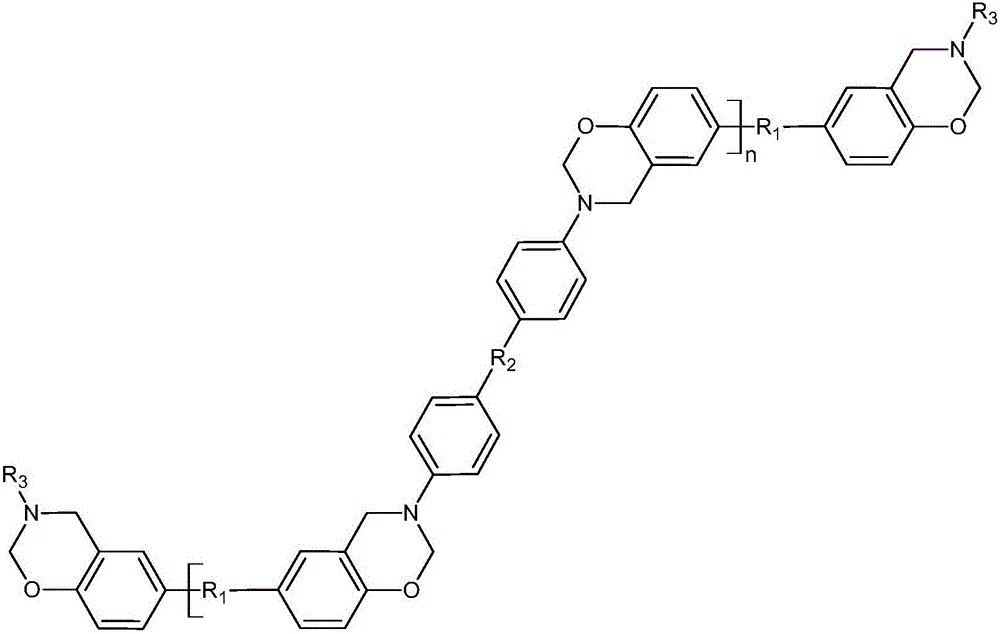
[0027] This example is a specific example of preparing Product 1, and the structural formula of Product 1 is shown below.
[0028]
[0029] Under room temperature conditions, weigh and / or measure bisphenol F (1mol), 4,4'-diaminodiphenyl ether (0.2mol) aniline (1.6mol) and 333.3g of formaldehyde aqueous solution with a mass concentration of 36%, add Catalyst NaOH 0.025mol, dissolved in a mixed solution of toluene (1000ml) and ethanol (20ml).
[0030] In this embodiment, the catalyst is 10% NaOH aqueous solution, and the catalyst accounts for 0.5% of the total mass of the reaction system; in the phenolic chemicals, amine compounds and aldehyde compounds, the molar ratio of the functional group phenolic hydroxyl group, amine group and aldehyde group is 1:1:2.
[0031] After the reaction system was stirred, it was heated under reflux at 60-120° C. for 4 hours. After heating under reflux, after vacuum distillation, cooling, washing, purification and drying, the product 1 was o...
Embodiment 2
[0034] This example is a specific example for preparing product 2, and the structural formula of product 2 is shown below.
[0035]
[0036] At room temperature, weigh and / or weigh 2 mol of bisphenol A, 0.2 mol of 4,4'-diaminodiphenylmethane, 4 mol of aniline and 9.6 mol of paraformaldehyde, add 0.2 mol of catalyst, dissolve in 1000 ml of toluene and 10 ml of ethanol in the mixed solution.
[0037] In this embodiment, the catalyst is 25% ammonia solution, and the catalyst accounts for 1% of the total mass of the reaction system; in the phenolic chemicals, amine compounds and aldehyde compounds, the molar ratio of the functional group phenolic hydroxyl group, amine group and aldehyde group is 1:1.05:2.4.
[0038] After the reaction system was stirred, it was heated under reflux at 60-120° C. for 4 hours. After heating under reflux, product 2 was obtained after vacuum distillation, cooling, washing, purification and drying. Butanone was added for dilution to obtain a 70% r...
Embodiment 3
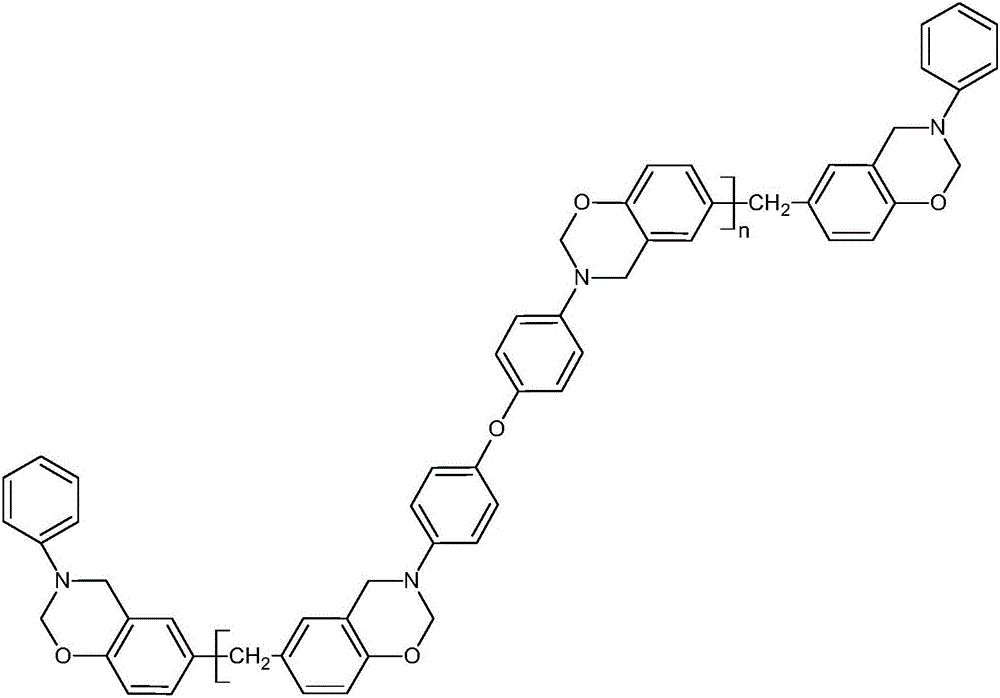
[0041] Examples are specific examples for preparing product 3, and the structural formula of product 3 is shown below.
[0042]
[0043] At room temperature, weigh and / or weigh 0.1 mol of bisphenol A, 0.09 mol of methylamine, 0.05 mol of 4,4'-diaminodiphenylmethane and 0.4 mol of paraformaldehyde, add 0.02 mol of catalyst, dissolve in toluene 100ml and 5ml of ethanol in a mixed solution.
[0044] In this embodiment, the catalyst is 25% ammonia solution, and the catalyst accounts for 1% of the total mass of the reaction system; in phenolic chemicals, amine compounds and aldehyde compounds, the molar ratio of the functional group phenolic hydroxyl group, amine group and aldehyde group It is 1.1:1:2.
[0045] After the reaction system was stirred, it was heated under reflux at 60-120° C. for 4 hours. After heating under reflux, product 3 was obtained after vacuum distillation, cooling, washing, purification and drying. Butanone was added for dilution to obtain a 70% resin s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com