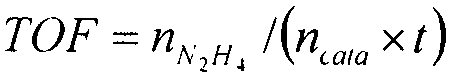Nickel-based catalyst for decomposing hydrazine hydrate and synthesis method of nickel-based catalyst
A hydrazine hydrate and catalyst technology, which is applied in difficult areas, can solve problems such as high dispersion, high cost, and insufficient technology, and achieve the effects of reducing agglomeration, improving catalytic activity, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The simple and easy preparation method of the described catalyst that is used to decompose hydrazine hydrate to prepare hydrogen comprises the following steps:
[0015] (1) Solution preparation: at room temperature and normal pressure, configure solution A with nickel source precursor and lanthanum source precursor, and configure solution B with reducing agent. The ratio is 1:x, where x=0.05-1;
[0016] (2) Catalyst preparation: quickly add solution B to solution A, stir magnetically for 10 minutes at room temperature and normal pressure, stop stirring after the reaction, transfer the solution to a centrifuge tube, and pour off the supernatant after centrifugation , adding ultrapure water for ultrasonic cleaning for 10 minutes, repeated centrifugation and cleaning for 3-4 times, and finally obtained a black precipitate, which was freeze-dried for 24 hours to obtain the desired catalyst sample for decomposing hydrazine hydrate to produce hydrogen.
[0017] The nickel so...
Embodiment 1
[0028] NiLa x Preparation of nanocatalysts:
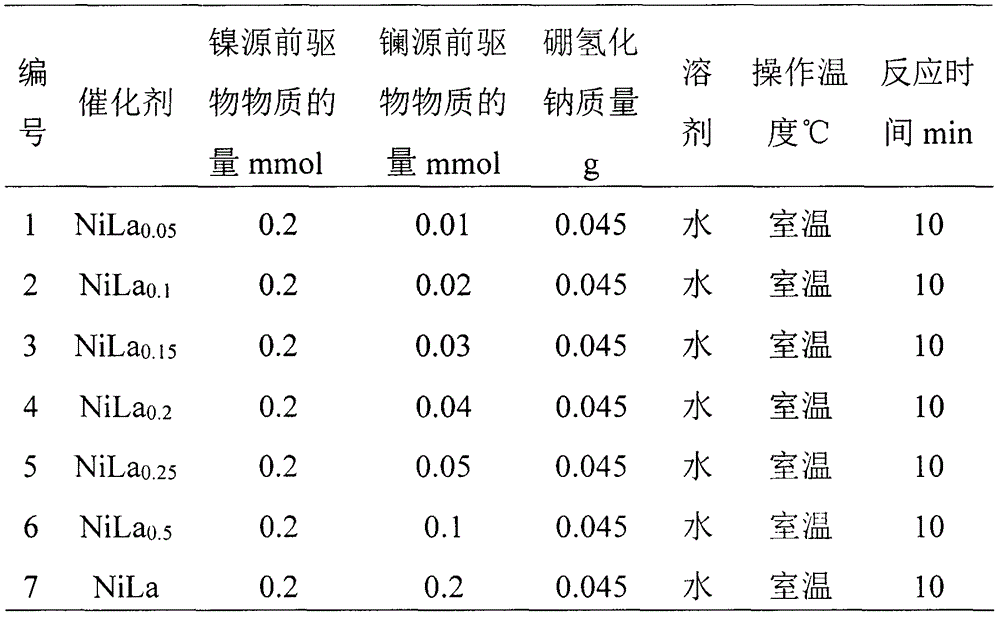
[0029] Get the solution containing 0.01, 0.02, 0.03, 0.04, 0.05, 0.1, 0.2mmol lanthanum chloride to obtain solution A in the 50ml round bottom flask containing 0.2mmol nickel chloride solution, get 0.045g of sodium borohydride (NaBH 4 ) was dissolved in 1.5ml deionized water to prepare solution B. Under the condition of magnetic stirring, solution B was quickly added to solution A, and a large amount of black suspended matter and bubbles were observed. After no more bubbles were generated, the solution in the round bottom flask was transferred to a centrifuge tube, centrifuged and ultrasonicated. Wash 3-4 times and freeze dry for 24 hours to get NiLa x Nanoparticles were made into catalysts numbered 1-7, as shown in Table 1.
[0030] Table 1 The nickel-lanthanum catalyst synthesized by different composition ratios
[0031]
Embodiment 2
[0033] Catalyst Activity Test
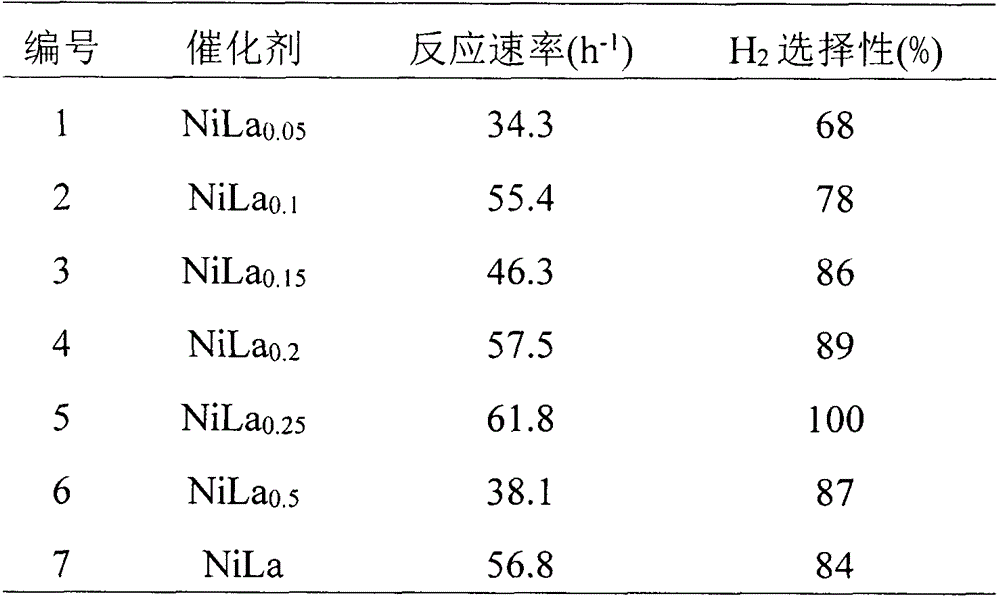
[0034] Catalyst evaluations of the present invention were performed in a closed drainage system. The experimental process is as follows: the reaction is carried out in a constant temperature water bath, 3 mL of deionized water and 50 mg of catalyst are added to a round bottom flask, 1 mL of 6mol / L NaOH solution is added, magnetically stirred for 1 min, and then 0.2 mL of hydrazine hydrate solution is added to the round bottom flask , start timing at the same time. The mass fraction of hydrazine hydrate used in the test is 50%. The gas produced by the catalytic decomposition of hydrazine hydrate absorbs the ammonia gas through the hydrochloric acid absorption device, and the rest is only hydrogen and nitrogen. The selectivity of the reaction can be calculated by reading the gas production. Compare the activity test results of catalysts with different composition ratios, as shown in Table 2.
[0035] Table 2 Comparison of catalyst activity test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com