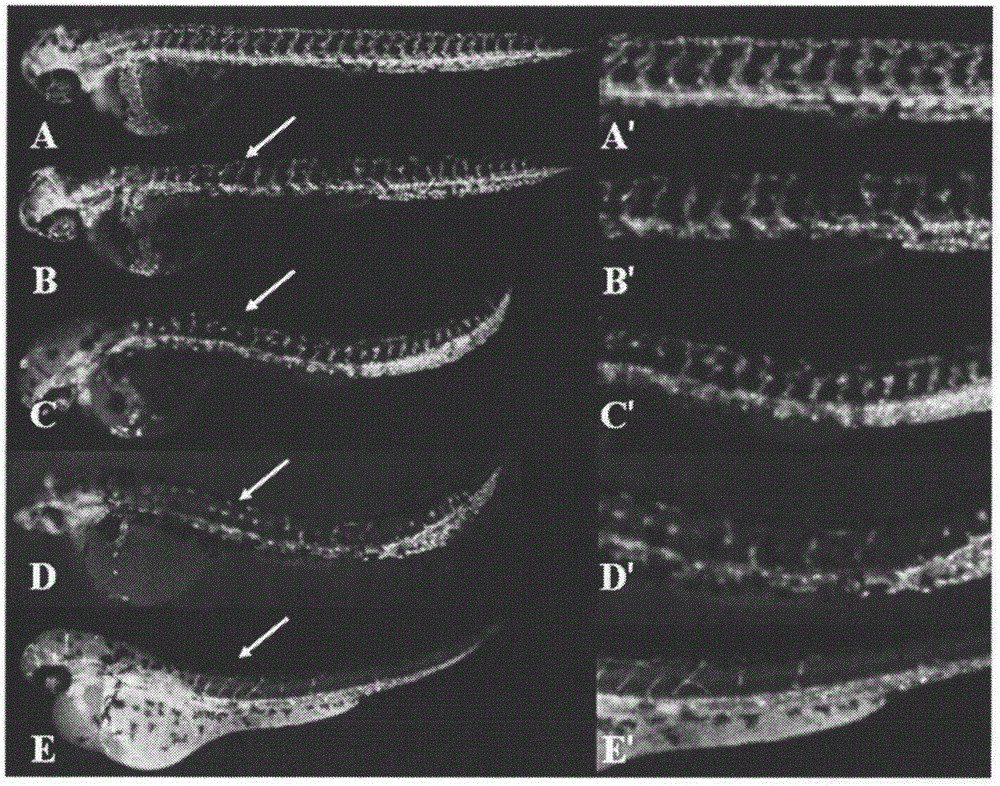
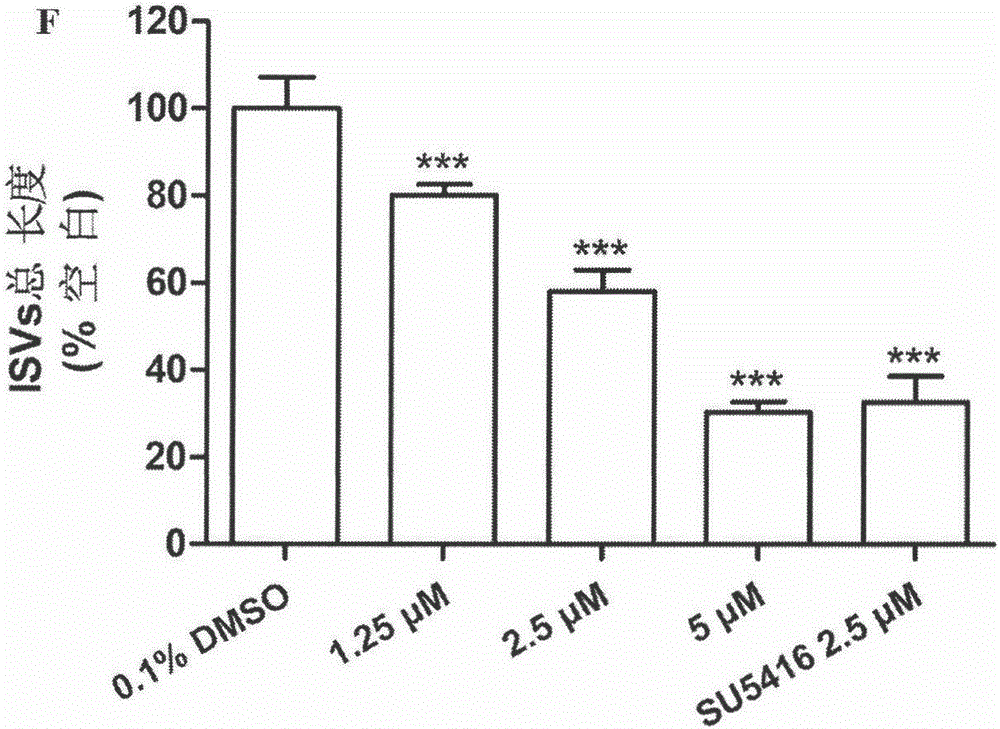
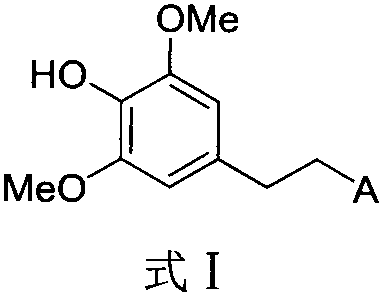
Halogen or nitrogen-containing group substituted bibenzyl analog, preparation method and uses thereof
A technology of nitrogen-containing groups and analogs, which is applied in the field of drug synthesis, can solve the problems of low content, single structure, and difficulty in obtaining large quantities, and achieve the effects of mild reaction conditions, simplified experimental process, and easy repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 4-hydroxyl-3,5-dimethoxy-3', the preparation of 4'-dichlorobibenzyl (compound 1)
[0035] a. 3,5-dimethoxy-4-acetoxybenzaldehyde
[0036]
[0037] Add 8.33 g (0.046 mol) of syringaldehyde (4-hydroxyl-3,5-dimethoxybenzaldehyde) powder in a 100 mL single-necked bottle, 10 mL of dichloromethane, and catalytically measure DMAP (4-dimethylaminopyridine), Stir to fully dissolve. Slowly add 4.75 mL (0.051 mol) of acetic anhydride dropwise, and react at room temperature for 1 h. Add water to wash away DMAP and acetic anhydride, wash 3 times in total, each time with 10 mL of water. Anhydrous Na 2 SO 4 The organic layer was dried and concentrated to recover dichloromethane to obtain 10.19 g of white crystals with a yield of 99.8%. It was identified as 3,5-dimethoxy-4-acetoxybenzaldehyde.
[0038] ESI-MS m / z: 225[M+H] + , The relative molecular mass is 224.
[0039] 1 H-NMR (300MHz, CDCl 3 )δ9.93 (1H, s, -CHO), 7.17 (2H, s, H-2, H-6), 3.92 (6H, s, 2×-OCH 3 ...
Embodiment 2
[0061] Example 2 Preparation of 4'-nitro-4-hydroxyl-3,5-dimethoxybibenzyl (compound 2)
[0062]
[0063] Add 200 mg (0.395 mmol) of (3,5-dimethoxy-4-acetoxybenzyl)-triphenylphosphine chloride and 60 mg (0.397 mmol) of p-nitrobenzaldehyde into a 50 mL single-necked bottle. Under the protection of argon, 3 mL of anhydrous THF was added, and stirred to form a light yellow suspension. Under ice-salt bath, 11 mg (0.458 mmol) of NaH was slowly added, and stirred overnight. TLC monitoring generates two new spots namely cis-trans isomerism stilbene ((Z / E)-4'-nitro-3,5-dimethoxy-4-acetoxy stilbene), concentrated sulfuric acid The color developed by ethanol was lavender, which proved that the reaction was successful. The reaction was terminated by adding ice water, extracted three times with dichloromethane, and the organic layer was anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure for later use.
[0064] Dissolve the concentrate in 5mL of methanol, add appropriate...
Embodiment 3
[0067] Example 3 The preparation of 4-hydroxyl-3,5-dimethoxy-4'-chlorobibenzyl (compound 3)
[0068]
[0069] Add 200 mg (0.395 mmol) of (3,5-dimethoxy-4-acetoxybenzyl)-triphenylphosphine chloride and 56 mg (0.398 mmol) of p-chlorobenzaldehyde into a 50 mL single-necked bottle. Add anhydrous THF 3mL under the protection of argon, and stir to form a white suspension. Under ice-salt bath, 11 mg (0.458 mmol) of NaH was slowly added, and stirred overnight. Two new spots generated by TLC monitoring were presumed to be cis-trans isomerism stilbene ((Z / E)-3,5-dimethoxy-4-acetoxy-4'-chlorostilbene), concentrated sulfuric acid The color developed by ethanol was lavender, which proved that the reaction was successful. The reaction was terminated by adding ice water, extracted three times with dichloromethane, and the organic layer was anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure for later use.
[0070] Dissolve the concentrate in 5mL of methanol, add appropriat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com