Preparing method of 3-hydroxypropionic acid ester
A hydroxypropionate and quinoline-based technology, applied in the field of preparation of 3-hydroxypropionate, can solve problems such as difficult separation of catalysts, and achieve the effects of mild catalytic reaction conditions, resolution of separation difficulties, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
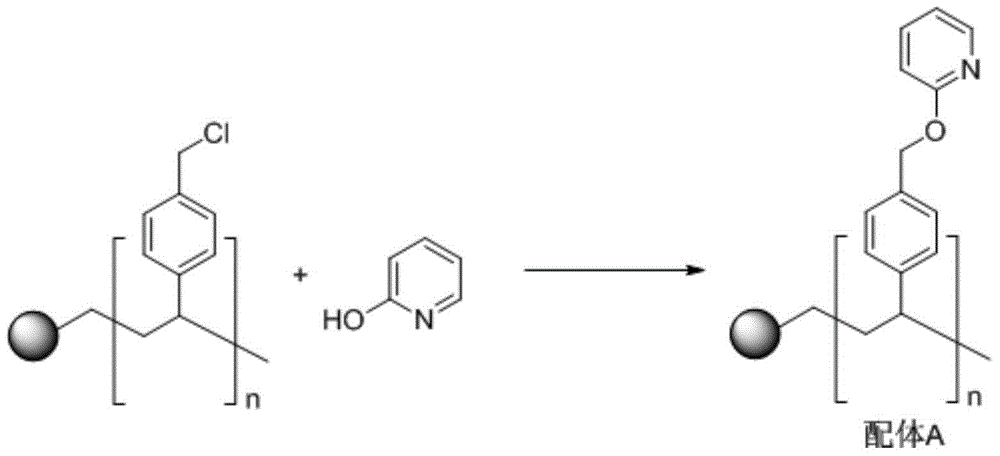
[0036] 1. Synthesis of ligand A
[0037]
[0038] Into a 100mL round-bottomed flask, add 5.0g of gel-type chloromethylated polystyrene resin (1% divinylbenzene cross-linked, chlorine content of 4.0mmol / g), 0.2mol of 2-hydroxypyridine, 0.2mol of Cesium carbonate and 200 mL of dioxane were reacted under reflux for 72 hours. The reaction was completed, suction filtration, washed the solid with 20 mL of dioxane for many times, then washed the solid with 20 mL of deionized water for many times, and finally washed the solid with 20 mL of ethanol and 20 mL of diethyl ether for many times, and vacuum-dried to obtain 5.87g of light yellow solid. It is ligand A (nitrogen content is 2.0 mmol / g).
[0039] 2. Synthesis of methyl 3-hydroxypropionate
[0040] 1.0 mmol of Co 2 (CO) 8 It was dissolved in 740 mmol of methanol, 1 g of ligand A was added, and the mixture was stirred at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, the reactor was...
Embodiment 2
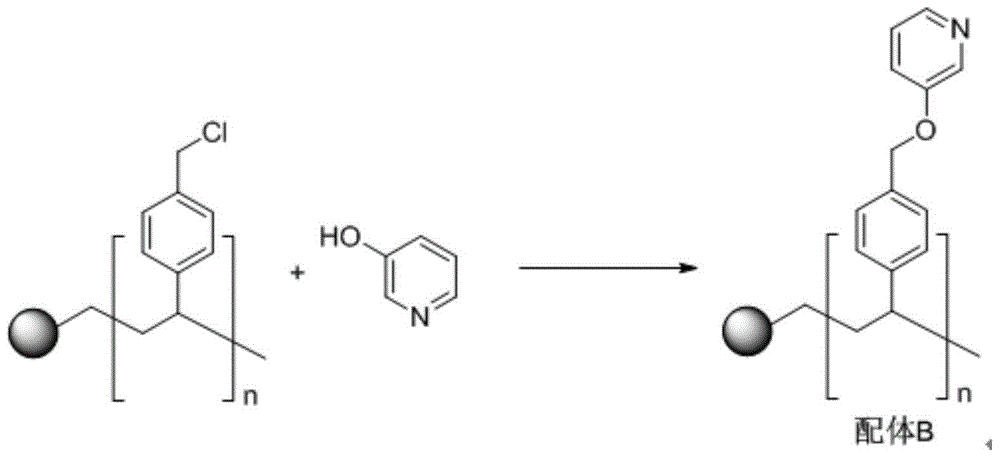
[0043] 1. Synthesis of ligand B
[0044]
[0045] In a 100mL round-bottomed flask, add 5.0g of gel-type chloromethylated polystyrene resin (1% divinylbenzene cross-linked, chlorine content of 4.0mmol / g), 0.2mol of 3-hydroxypyridine, 0.2mol of Cesium carbonate and 200 mL of dioxane were reacted under reflux for 72 hours. The reaction was completed, suction filtration, washed the solid with 20 mL of dioxane for many times, then washed the solid with 20 mL of deionized water for many times, and finally washed the solid with 20 mL of ethanol and 20 mL of diethyl ether for many times, and vacuum-dried to obtain 5.87g of light yellow solid. It is ligand B (nitrogen content is 2.2mmol / g).
[0046] 2. Synthesis of methyl 3-hydroxypropionate
[0047] 1.0 mmol of Co 2 (CO) 8 It was dissolved in 740 mmol of methanol, 0.91 g of ligand B was added, and the mixture was stirred at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, the reactor was p...
Embodiment 3
[0050] 1. Synthesis of ligand C
[0051]
[0052] In a 100mL round-bottomed flask, add 5.0g of gel-type chloromethylated polystyrene resin (1% divinylbenzene cross-linked, chlorine content of 4.0mmol / g), 0.2mol of 4-hydroxypyridine, 0.2mol of Cesium carbonate and 200 mL of dioxane were reacted under reflux for 72 hours. The reaction was completed, suction filtration, washed the solid with 20 mL of dioxane for many times, then washed the solid with 20 mL of deionized water for many times, and finally washed the solid with 20 mL of ethanol and 20 mL of diethyl ether for many times, and vacuum-dried to obtain 5.87g of light yellow solid. It is ligand C (nitrogen content is 2.5mmol / g).
[0053] 2. Synthesis of methyl 3-hydroxypropionate
[0054] 1.0 mmol of Co 2 (CO) 8 It was dissolved in 740 mmol of methanol, 0.8 g of ligand C was added, and the mixture was stirred at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, the reactor was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com