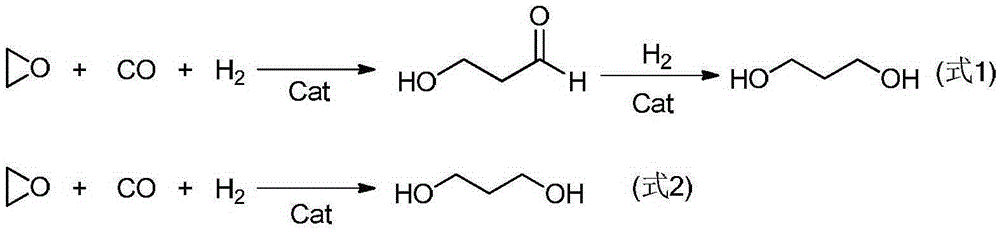
Preparation method of 3-hydroxy-propionaldehyde
A technology of hydroxypropionaldehyde and cobalt carbonyl, which is applied in the field of preparation of 3-hydroxypropionaldehyde, can solve the problems such as the difficulty of recycling the catalyst, and achieves the effects of mild catalytic reaction conditions, solving difficulty in separation, and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
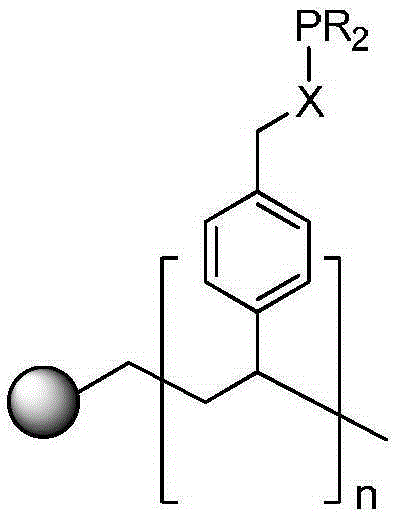
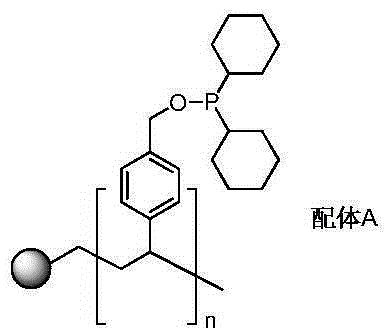
[0034] Add 5.0 g of gel-type hydroxymethylated polystyrene resin (1% divinylbenzene cross-linked, hydroxyl content of 3.5 mmol / g to 4.5 mmol / g) and 225 mmol of dicyclohexyl chloride in a 100 mL reaction tube. Phosphine, 50mol of triethylamine and 200mL of dry toluene; replace the air in the reaction tube with high-purity nitrogen three times, and react at 45°C for 72 hours under nitrogen atmosphere. After the reaction was completed, the solid was filtered with suction, washed with 20 mL of toluene and 20 mL of dichloromethane for several times, and vacuum-dried to obtain 6.40 g of light yellow solid which was Ligand A (the phosphorus content was 1.1 mmol / g).
[0035]
[0036] 1.0mmol of Co 2 (CO) 8 Dissolve in 30mL of chlorobenzene, add 0.91g of Ligand A, and stir at room temperature for 4 hours; then transfer the solution to a 100mL reactor, purging the reactor with high-purity nitrogen three times, adding 50mmol of ethylene oxide, Add synthesis gas (the volume ratio of ...
Embodiment 2
[0039] Add 5.0 g of gel-type hydroxymethylated polystyrene resin (1% divinylbenzene cross-linked, hydroxyl content of 3.5 mmol / g to 4.5 mmol / g) into a 100 mL reaction tube, 225 mmol of diphenyl chloride Phosphine, 50 mol of triethylamine and 200 mL of dry toluene; replace the air in the reaction tube with high-purity nitrogen three times, and react at 45°C for 72 hours under a nitrogen atmosphere. After the reaction was completed, suction filtered, the solid was washed several times with 20 mL toluene and 20 mL dichloromethane respectively, and vacuum-dried to obtain 7.10 g of a light yellow solid which was Ligand B (with a phosphorus content of 1.6 mmol / g).
[0040]
[0041] 1.0mmol of Co 2 (CO) 8 Dissolve in 30mL of 1,3-dioxolane, add 1.6g of Ligand B, and stir at room temperature for 4 hours; then transfer the solution to a 100mL reactor, purging the reactor with high-purity nitrogen three times, adding 50mmol of ethylene oxide was added into synthesis gas (the volume ...
Embodiment 3
[0043] Add 5.0 g of gel-type hydroxymethylated polystyrene resin (1% divinylbenzene cross-linked, hydroxyl content of 3.5 mmol / g to 4.5 mmol / g) and 225 mmol of bis(4-formaldehyde) into a 100 mL reaction tube. phenyl) phosphine chloride, 50 mol of triethylamine and 200 mL of dry toluene; replace the air in the reaction tube with high-purity nitrogen three times, and react at 45° C. for 72 hours under a nitrogen atmosphere. After the reaction was completed, the solid was filtered with suction, washed with 20 mL of toluene and 20 mL of dichloromethane for several times, and dried in vacuo to obtain 7.30 g of a yellow solid which was Ligand C (with a phosphorus content of 1.48 mmol / g).
[0044]
[0045] 1.0mmol of Co 2 (CO) 8 Dissolve in 30mL of methyl tert-butyl ether, add 1.4g of ligand C, and stir at room temperature for 4 hours; then transfer the solution to a 100mL reaction kettle, purging the reaction kettle with high-purity nitrogen three times, and adding 50mmol of ring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com