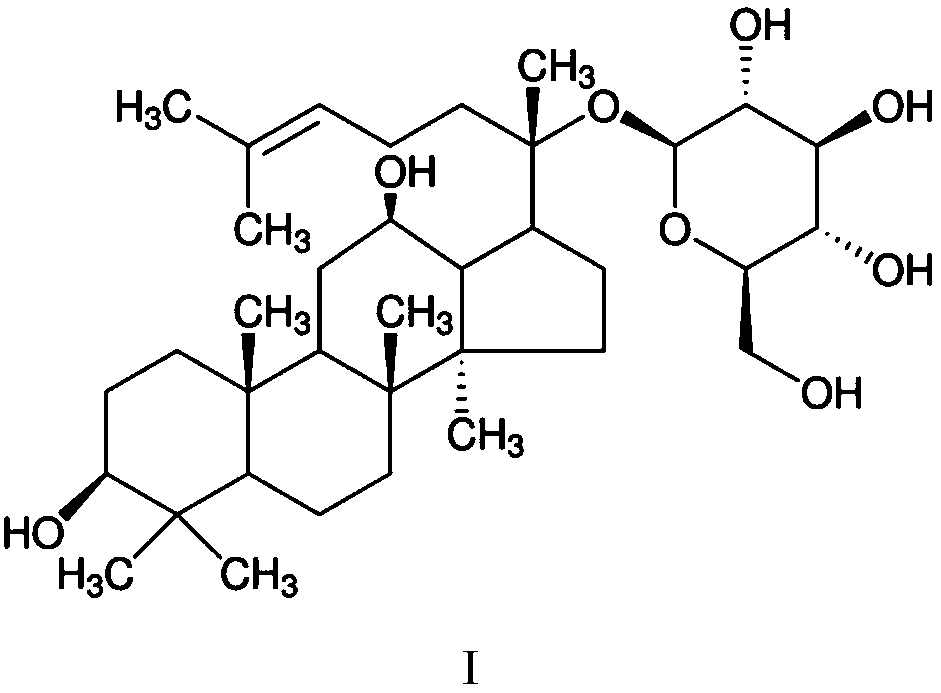
Glycosyltransferase mutants
A technology of glycosyltransferases and mutants, applied in the direction of transferases, enzymes, genetic engineering, etc., can solve problems such as low yield, harsh growth environment requirements, and inability to meet social needs, and achieve improved catalytic activity and enzyme-catalyzed reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 Construction of wild-type UGT1 expression strain
[0065] Synthesize the gene sequence encoding UGT1 as SEQ ID NO: 2, design restriction sites NdeI and BamHI at both ends, clone it into the corresponding site on the pSH plasmid, obtain the recombinant plasmid pSH-UGT1, and then transform it into E. coli expression host by electroporation For BL21(DE3) competent cells, coat the LB medium plate containing kanamycin, cultivate overnight at 37°C, pick a single colony, inoculate it into a test tube containing LB medium, cultivate overnight, collect the bacteria by centrifugation, and extract The plasmid was confirmed to be correct by gene sequencing, and a recombinant genetically engineered strain BL21(DE3) / pSH-UGT1 expressing wild-type UGT1 was obtained.
[0066] The amino acid sequence was determined to be UGT1.
[0067] Select a single clone from a plate containing UGT1 engineered strains, inoculate into 5mL LB medium, and culture at 37°C; inoculate 1% v / v i...
Embodiment 2
[0068] Example 2 Error-prone PCR method constructs UGT1 random mutation point library
[0069] Using pSH-UGT1 as a template, an error-prone PCR technique was used to construct a random mutant library.
[0070] Forward primer UGT1-Nde-F: 5'-CAT ATGAAATCTGAATTAATCTTTT-3',
[0071] Reverse primer UGT1-Bam-R: 5'-GGATCC CTACATAATTTCTTCGAATAAT-3'.
[0072] 50μL error-prone PCR reaction system includes: 50ng plasmid template pSH-UGT1, 30pmol a pair of primers UGT1-Nde-F and UGT1-Bam-R, 1X Taq buffer, 0.2mM dGTP, 0.2mM dATP, 1mM dCTP, 1mM dTTP, 7mM MgCl 2 , (0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM) MnCl 2 , 2.5 units of Taq enzyme (fermentas). The PCR reaction conditions were: 95°C for 5 minutes; 94°C for 30s, 55°C for 30s, 72°C for 2min / kbp; 30 cycles; 72°C for 10min. The 2.0kb random mutation fragment was recovered from the gel as a large primer, and MegaPrimer PCR was performed with KOD-plus DNA polymerase: 94°C for 5min; 98°C for 10s, 60°C for 30s, 68°C for 2min / kbp, 25 cycles; 68°...
Embodiment 3
[0073] High-throughput screening of embodiment 3 UGT1 mutant library
[0074] 3.1 Select the transformant from the UGT1 mutant library and inoculate it into a 96-well deep-well culture plate containing 700 μL of LB medium containing 100 μg / mL kanamycin. After culturing at 37°C for 6 hours, add a final concentration of 0.1 mM IPTG Afterwards, the temperature was lowered to 25°C and cultured overnight. Centrifuge at 5000rpm for 10min, discard the supernatant, freeze at -70°C for 1h, and thaw at room temperature for 30min. Add 200 μL of lysate (components are 20mM Tris-HCl, pH8.0, 2mM 1,4-dithiothreitol, 4000U lysozyme), resuspend the bacteria, incubate at 37°C for 1 hour, and centrifuge thoroughly to obtain supernatant enzyme liquid.
[0075] Use the enzyme solution obtained above to transfer to a 96-well plate for enzyme reaction, and perform screening according to the method of "High-throughput screening reaction of glycosyltransferase mutants" described above, using wild-ty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com