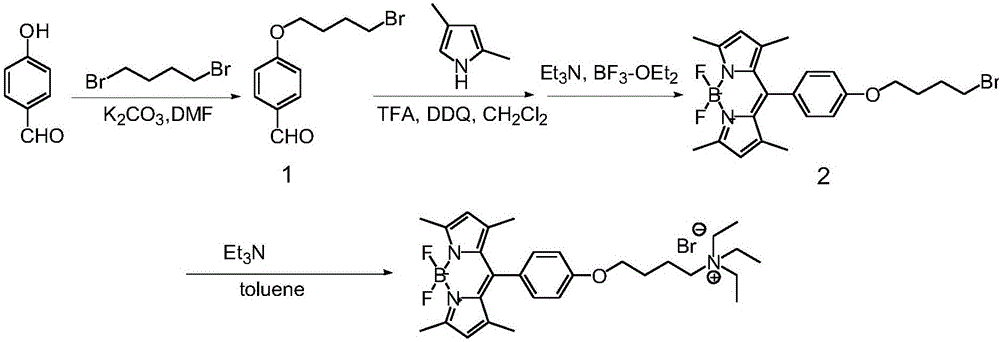
Mitochondria target fluorescence probe, as well as preparation method and application thereof
A fluorescent probe and fluorescent molecular probe technology, applied in the field of light analysis and detection, can solve the problems of weak resistance to photobleaching, high cytotoxicity, unsuitable for long-term mitochondrial imaging, etc., and achieve strong resistance to photobleaching and cytotoxicity. Low, low impact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of fluorescent organic molecule (TEA-BODIPY) with mitochondrial targeting function
[0034] Dissolve p-hydroxybenzaldehyde (1.22g, 10mmol) and 1,4-dibromobutane (4.30g, 20mmol) in 20mL of anhydrous N,N-dimethylformamide (DMF), add anhydrous potassium carbonate (K 2 CO 3 ) (2.07g, 15mmol) was used as a base, reacted at room temperature to obtain a crude product, and passed through a chromatographic column to obtain compound 1. The eluent was n-hexane-acetone mixture of n-hexane / acetone (v / v)=8 / 1 , the structural formula of compound 1 is
[0035] Compound 1 (0.512g, 2mmol) and 2,4-dimethylpyrrole (0.380g, 4mmol) were dissolved in 200mL of dry dichloromethane, under the protection of nitrogen, a small amount of trifluoroacetic acid (TFA) was used as Catalyst, after stirring at room temperature for 12 hours, add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (2.27g, 20mmol), then continue stirring at room temperature for 2 hours, stirring Add 5...
Embodiment 2
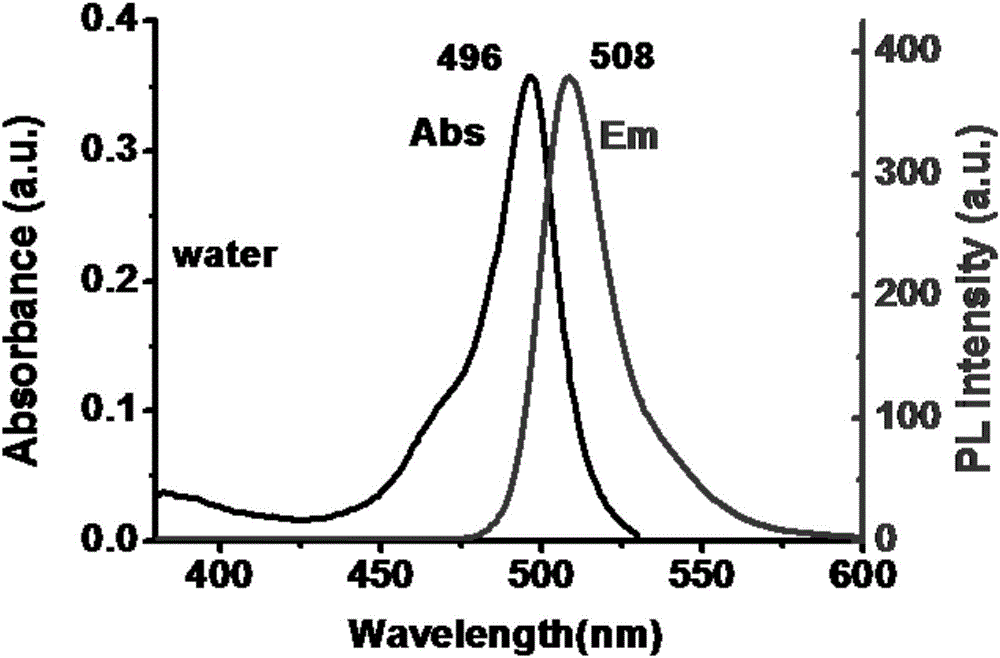
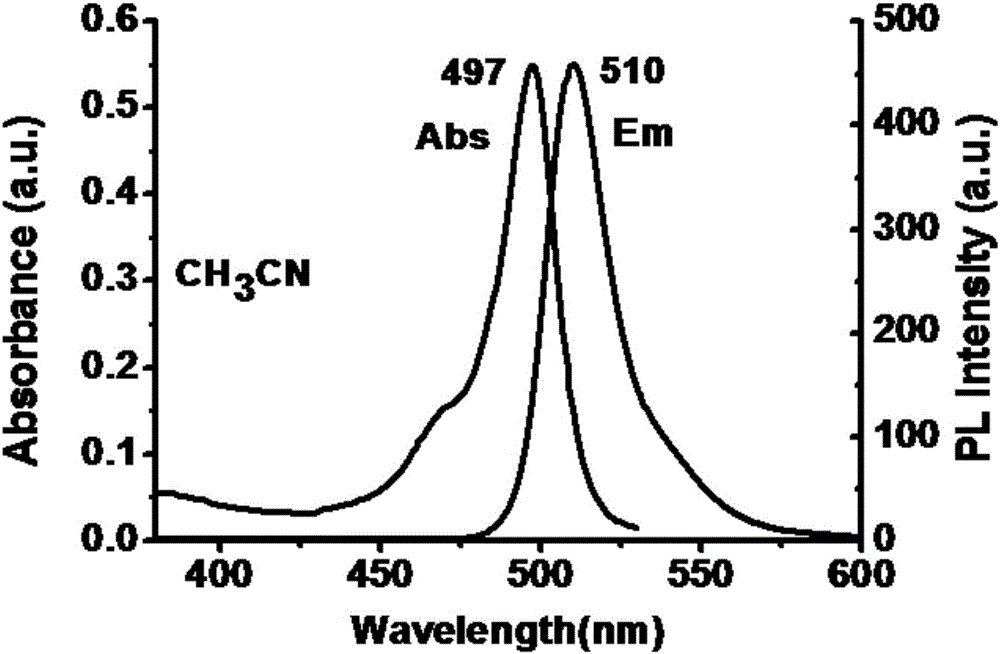
[0037] Example 2: Absorption and emission spectrum tests of mitochondrial targeting fluorescent probe TEA-BODIPY in different polar solutions
[0038] The spectrum test concentration that the present invention adopts is 10 μ M, and the solvent of test is aqueous solution and acetonitrile solution, and the absorption and emission spectra of TEA-BODIPY in different solvents are as follows: figure 2 with image 3 shown. The polarity of the solvent has little change on the absorption and emission spectra of the probe, and its Stokes shift has almost no change. It can be seen that the polarity of the solvent has little effect on the probe TEA-BODIPY.
Embodiment 3
[0039] Example 3: Test of fluorescence intensity of mitochondrial targeting fluorescent probe TEA-BODIPY under different pH conditions
[0040] The spectral test concentration that the present invention adopts is 10 μ M, and the test of the fluorescence intensity of TEA-BODIPY under pH condition is as follows Figure 4 shown. The pH has little effect on the fluorescence intensity of the probe, indicating that the probe can be used normally under the condition of physiological pH of the cell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com