Preparation and stripping methods of lamellar zinc hydroxide and zinc oxide nanocones
A zinc hydroxide and nano-cone technology, applied in the direction of zinc oxide/zinc hydroxide, etc., can solve the problems of little research on the synthesis and stripping of layered zinc hydroxide nanomaterials, high energy consumption, and complexity, and achieve energy saving, The effect of high quality and controllable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
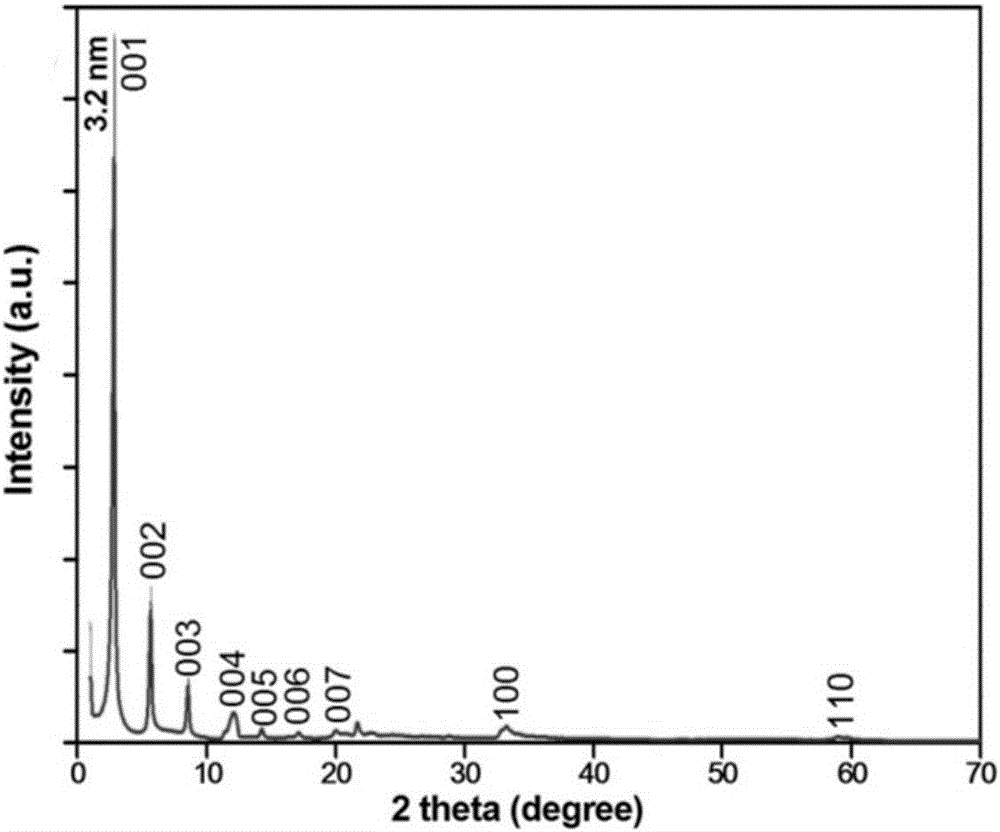
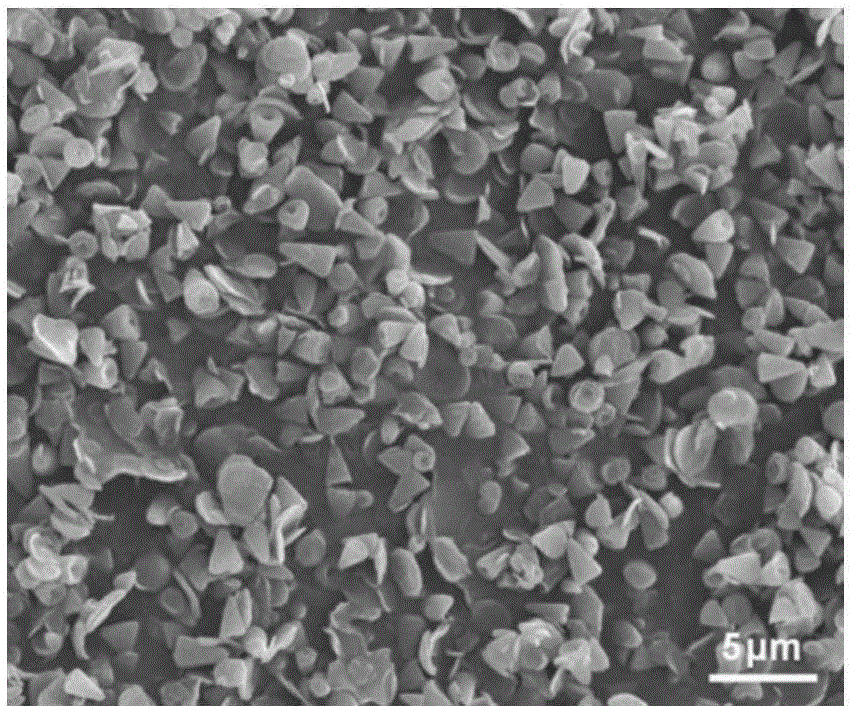
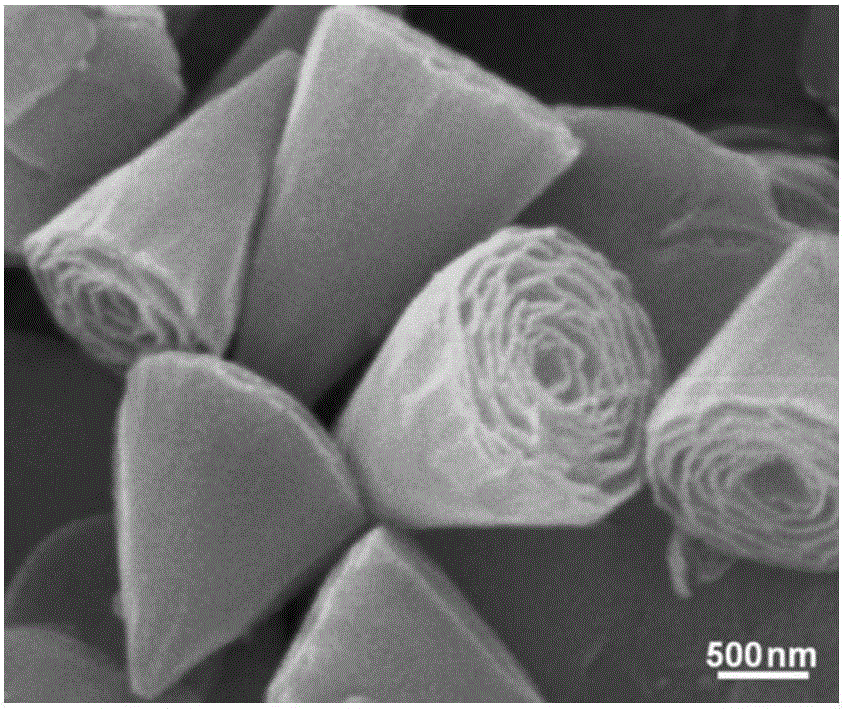
[0040] Weigh 1.25mmol (0.17g) of zinc chloride and dissolve it in a beaker filled with 150ml of pure water, then weigh 3.125mmol (0.9g) of sodium lauryl sulfate and dissolve it in pure water, stir evenly for 10min, then add 8.75mmol (1.225g) of hexamethylenetetramine, continue to stir for 15 minutes, seal the mouth of the beaker with plastic wrap, move the beaker into a 95°C water bath, and keep it warm for 8 hours. The product obtained by the reaction was centrifuged, washed and dried to obtain a white powder. Such as figure 1 Shown product is identified as layered zinc hydroxide through X-ray powder diffraction; Carry out morphology analysis to layered zinc hydroxide with scanning electron microscope (SEM), from figure 2 , 3 It can be seen that its morphology is a nanocone with a length of about 1.5 μm; the morphology of layered zinc hydroxide was analyzed by transmission electron microscopy (TEM), and the Figure 4 It can be seen that its morphology is a layered nanocon...
Embodiment 2
[0042] Weigh 1.25mmol (0.17g) of zinc chloride and dissolve it in a beaker filled with 150ml of pure water, stir evenly for 10 minutes, add 8.75mmol (1.225g) of hexamethylenetetramine, continue stirring for 15 minutes and seal it with plastic wrap Move the beaker into a 95°C water bath and keep it warm for 8 hours. The product obtained by the reaction was centrifuged, washed and dried to obtain a white powder. Its morphology was analyzed by scanning electron microscope (SEM). Figure 5 It can be seen that its morphology is ZnO nanorods.
Embodiment 3
[0044] Weigh 1.25mmol (0.17g) of zinc chloride and dissolve it in a beaker containing 150ml of pure water, then weigh 1mmol (0.288g) of sodium lauryl sulfate and dissolve it in pure water, stir evenly for 10min and add 8.75mmol ( 1.225g) of hexamethylenetetramine, continue to stir for 15 minutes, seal the mouth of the beaker with plastic wrap, move the beaker into a 95°C water bath, and keep it warm for 8 hours. The product obtained by the reaction was centrifuged, washed and dried to obtain a white powder. Its morphology was analyzed by scanning electron microscope (SEM). Image 6 It can be seen that its morphology is layered zinc hydroxide nanosheets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com