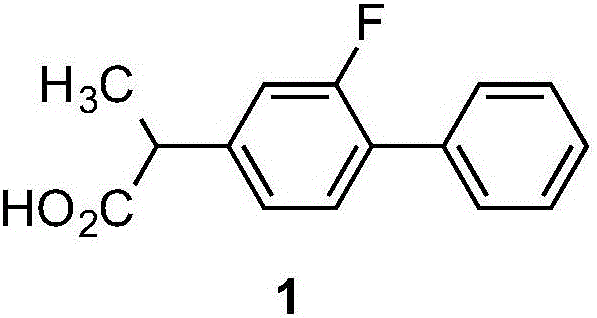
Flurbiprofen synthesis method
A technology of flurbiprofen and its synthesis method, which is applied in the field of drug synthesis, can solve the problems of high cost, great harm to the environment and operators, and low yield, and achieve the effects of less three wastes, high atom utilization rate, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
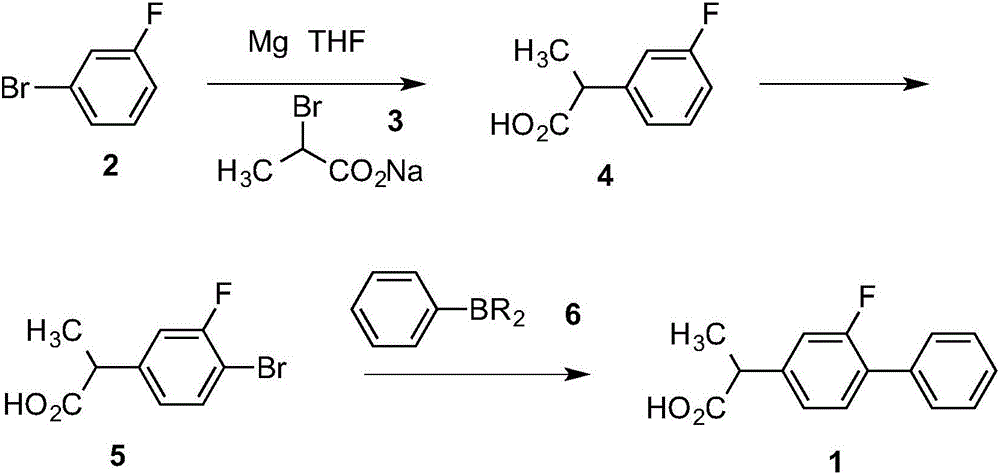
[0035] ① Preparation of Grignard reagent: It is prepared by reacting 3-fluorobromobenzene and magnesium chips in THF, wherein the molar ratio of 3-fluorobromobenzene and magnesium chips is 1: (1-2).
[0036] ②Coupling reaction: Add sodium 2-bromopropionate to the Grignard reagent of 3-fluorobromobenzene under the condition of -10~70℃, gradually heat to reflux after adding, react for 1-1.5h, react Finish.
[0037] ③Acidification reaction: Cool the reaction solution of the coupling reaction to 0°C, slowly add hydrochloric acid, the temperature does not exceed 20°C, stir naturally for 30-40min after adding, then heat to 50-60°C and stir for 1-1.5h, then Separate the liquid, extract the aqueous layer twice with ethyl acetate, combine the organic phase and the extract, evaporate the solvent in a water bath under reduced pressure until solids appear, add toluene, stir and cool down to -10°C, and filter the resulting white solid with suction Drying affords 2-(3-fluorophenyl)propanoi...
Embodiment 1
[0042] (1) Preparation of 2-(3-fluorophenyl) propionic acid (4)
[0043] In a 1000ml dry four-neck flask, add 14.4g (0.6mol) of magnesium chips, 50ml of THF, and two iodine pellets, stir and heat to 50°C in a nitrogen atmosphere, and mix 87.5g (0.5mol) of 3-fluorobromobenzene with 370ml of THF , and then drop 20ml of the mixed solution first. After the initiation is confirmed and the reaction is stable, the remaining mixed solution is added dropwise at 50-60°C. After the dropping, keep stirring for 1 hour.
[0044] Cool the reaction solution to 0°C, add 96.3g (0.55mol) of sodium 2-bromopropionate at a temperature not exceeding 10°C, and gradually heat to reflux for 1 hour after the addition, and the reaction ends.
[0045]Cool the reaction solution to 0°C, slowly add 400ml of 5mol / L hydrochloric acid, the temperature does not exceed 20°C, stir naturally for 30min after the addition, then heat to 50°C and stir for 1h.
[0046] Separate the liquid, extract the aqueous layer twi...
Embodiment 2
[0054] (1) Preparation of 2-(3-fluorophenyl) propionic acid (4)
[0055] In a 1000ml dry four-neck flask, add 14.4g (0.6mol) of magnesium chips, 50ml of THF, and two iodine pellets, stir and heat to 50°C in a nitrogen atmosphere. Mix 87.5g (0.5mol) of 3-fluorobromobenzene with 370ml THF, and then drop 20ml of the mixed solution first. After confirming the trigger and the reaction is stable, add the remaining mixed solution dropwise at 52-58°C, and keep stirring for 1 hour after dropping. .
[0056] Cool the reaction solution to 0°C, add 105g (0.6mol) sodium 2-bromopropionate at a temperature not exceeding 10°C, and gradually heat to reflux for 1 hour after the addition, and the reaction ends.
[0057] Cool the reaction solution to 0°C, slowly add 400ml of 5mol / L hydrochloric acid, the temperature does not exceed 20°C, stir naturally for 32min after the addition, then heat to 52°C and stir for 1h.
[0058] Separate the liquid, extract the aqueous layer twice with 200ml of eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com