Single crystal nickel cobalt lithium manganate cathode material and preparation method thereof and lithium ion battery
A technology for nickel-cobalt manganate lithium and lithium-ion batteries, which is applied in battery electrodes, secondary batteries, circuits, etc., and can solve the problems of low capacity and low rate performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
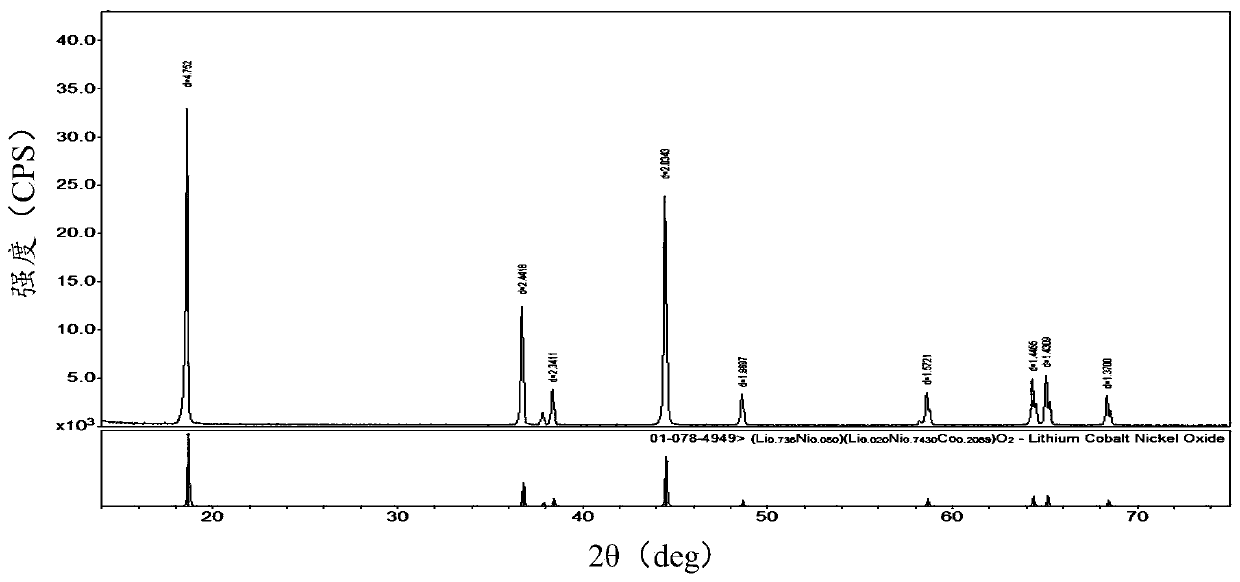
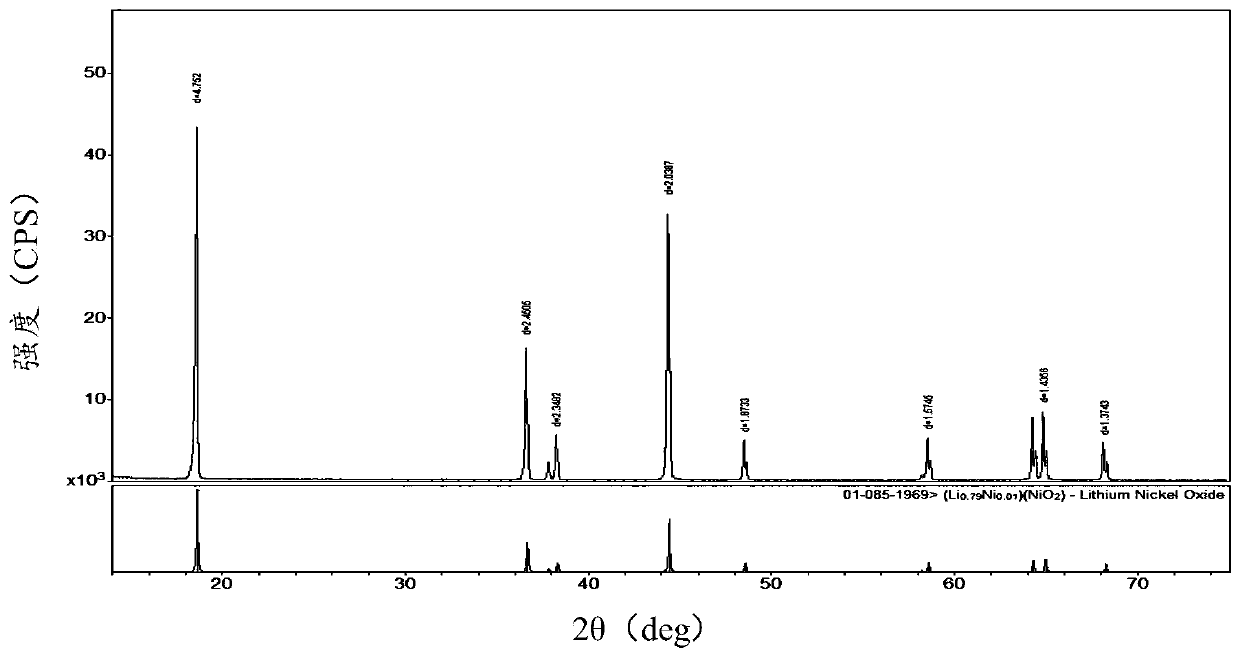
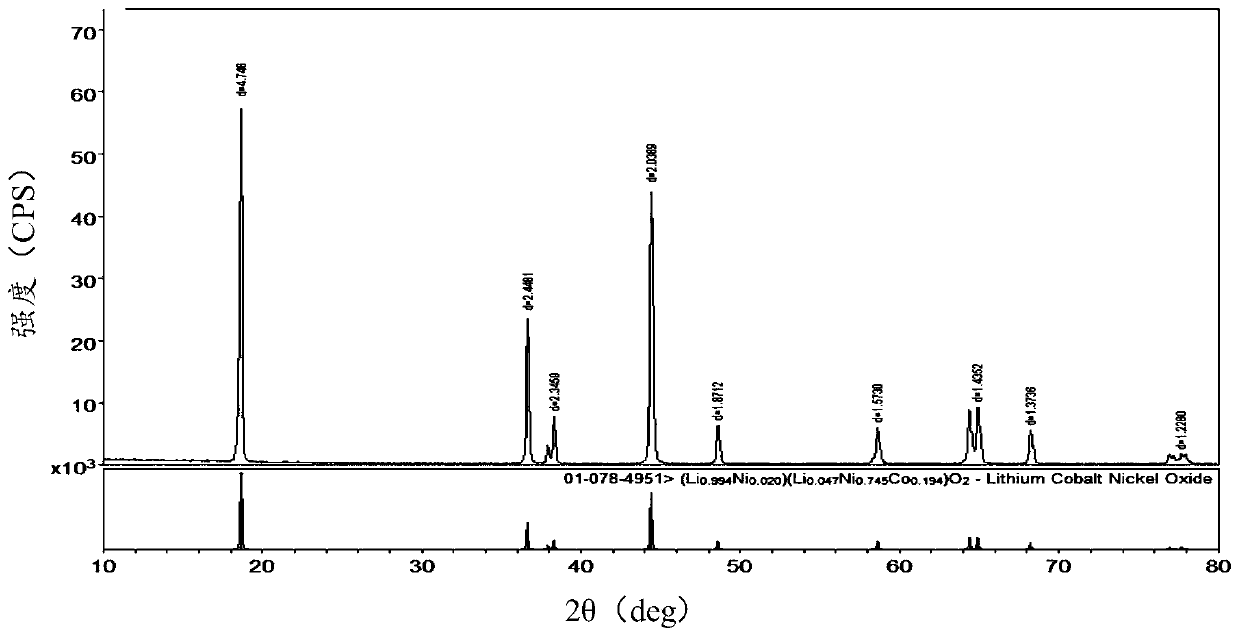
Image
Examples
preparation example Construction
[0052] The present invention also provides a method for preparing the above-mentioned single crystal nickel cobalt lithium manganese oxide positive electrode material, comprising the following steps:
[0053] S1) Mix and react nickel salt, cobalt salt, manganese salt, alkali and complexing agent to obtain nickel cobalt manganese hydroxide precursor; The molar ratio is x:y:(1-x-y); 0.3≤x≤0.75, 0.2≤y≤0.3;
[0054] S2) Mix and sinter the nickel-cobalt-manganese hydroxide precursor and the first lithium salt to obtain a substrate; the molar ratio of lithium ions in the first lithium salt to nickel ions in the nickel-cobalt-manganese hydroxide precursor is: 1: x;
[0055] Or the nickel-cobalt-manganese hydroxide precursor, the first lithium salt and the M-containing compound are mixed and sintered to obtain the substrate; M is Ti, Mg, Al, V, Cr, Zr, Ba, La, Ce, Sn elements One or more; the molar ratio of lithium ions in the first lithium salt, nickel ions in the nickel-cobalt-man...
Embodiment 1
[0076] 1.1 Dissolve nickel sulfate, cobalt sulfate, and manganese sulfate in deionized water at a molar ratio of Ni, Co, and Mn of 1:1:1 to prepare a mixed salt solution with a total metal cation concentration of 1mol / L, and stir to make it fully Mix well to obtain a mixed salt solution.
[0077] 1.2 Prepare 5mol / L sodium hydroxide solution and 5mol / L ammonia water respectively, then pump the prepared mixed salt solution into the reaction kettle at a rate of 150L / h and stir it, while controlling the sodium hydroxide and ammonia water The flow rate of the aqueous solution is such that the pH value of the mixed solution is 10.5, and the temperature of the reaction kettle is controlled at 50°C during the process; the stirring speed is 300rpm, and the mixed salt solution to be prepared is added into the reaction kettle and continued to stir and age for 16h, and then the obtained The solid-liquid mixture was separated by centrifugal filtration, the solid was washed with deionized w...
Embodiment 2
[0084] 2.1 Dissolve nickel nitrate, cobalt nitrate, and manganese nitrate in deionized water at a molar ratio of Ni, Co, and Mn of 5:2:3 to prepare a salt solution with a total metal cation concentration of 1.6mol / L, and stir to fully Mix well to obtain a mixed salt solution.
[0085] 2.2 Prepare 4mol / L sodium hydroxide solution and 4mol / L ammonia water respectively, then pump the prepared mixed salt solution into the reaction kettle at a rate of 160L / h and stir it, while controlling the sodium hydroxide and ammonia water The flow rate of the aqueous solution is such that the pH value of the mixed solution is 11.2, and the temperature of the reaction kettle is controlled at 60°C during the process; the stirring speed is 400rpm. After all the salt solution to be prepared is added to the reaction kettle, continue to stir and age for 16h, and then the obtained solid-liquid mixture After separation by centrifugal filtration, the solid was washed with deionized water until neutral,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com