Dimetridazole encapsulated preparation and preparation method thereof
A nitazole package and preparation technology, which is applied in the directions of capsule delivery, pharmaceutical formulations, microcapsules, etc., can solve the problems of low bioavailability, strong irritation, weak stability, etc., and achieves improved feed intake, good palatability, Stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
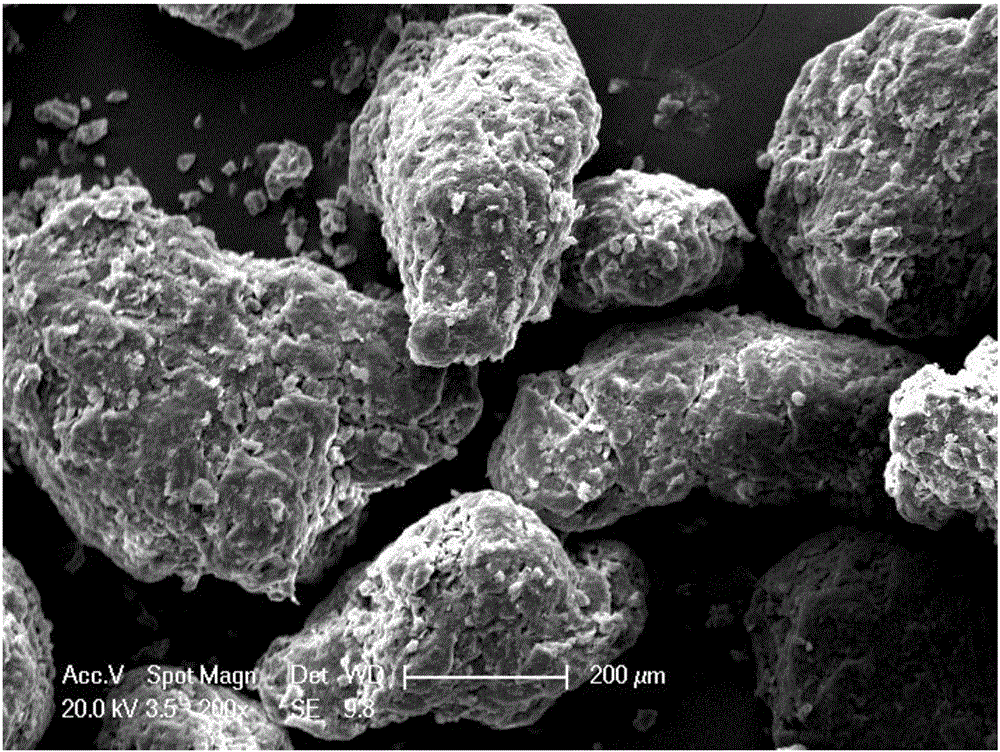
[0015] Add the original powder of Dimenidazole into stearic acid oil containing 3% by mass of calcium stearate and 1% of propylene glycol fatty acid ester in a mass ratio of 1:1, emulsify and shear at high speed for 40 minutes, The temperature is controlled at 70-80°C; the obtained material is transferred to 4 times the volume of an aqueous solution containing 1% chitosan, 0.5% sodium alginate and 1% sucrose ester, and the temperature is controlled at 70-80°C. Stir at a low speed of 200rpm for 30 minutes and then start to cool down until the encapsulated preparation of demenidazole in the solution is completely precipitated; stop stirring, filter and dehydrate the above materials through a 100-mesh sieve, and dry them in vacuum at low temperature to make encapsulated demenidazole preparation. The particle diameter range of this preparation is in 150 μ m-300 μ m, and the rate of recovery (the ratio of the weight of Dimenidazole encapsulated preparation and the total weight of f...
Embodiment 2
[0017] Add the original powder of dimenidazole into the stearic acid oil containing 5% calcium stearate and 0.8% propylene glycol fatty acid ester in a mass ratio of 1:10, high-speed emulsifying and shearing stirring for 20 minutes, The temperature is controlled at 70-80°C; the obtained material is transferred to 5 times the volume of an aqueous solution containing 1% chitosan, 0.5% sodium alginate and 1% sucrose ester, and the temperature is controlled at 70-80°C. Stir at a low speed of 200rpm for 30 minutes and then start to cool down until the encapsulated preparation of demenidazole in the solution is completely precipitated; stop stirring, filter and dehydrate the above materials through a 100-mesh sieve, and dry them in vacuum at low temperature to make encapsulated demenidazole preparation. The particle size range of the encapsulated preparation is 200 μm-500 μm, the recovery rate is 95.3%, the encapsulation rate is 97.6%, and the drug loading is 8.7%.
Embodiment 3
[0019] Add the original powder of Dimenidazole into the stearic acid oil containing 2% calcium stearate and 1.5% propylene glycol fatty acid ester in a mass ratio of 2:1, high-speed emulsifying and shearing stirring for 60 minutes, The temperature is controlled at 70-80°C; the obtained material is transferred to 4 times the volume of an aqueous solution containing 2% chitosan, 1% sodium alginate and 1.5% sucrose ester, and the temperature is controlled at 70-80°C. Stir at a low speed of 200rpm for 30 minutes and then start to cool down until the encapsulated preparation of demenidazole in the solution is completely precipitated; stop stirring, filter and dehydrate the above materials through a 100-mesh sieve, and dry them in vacuum at low temperature to make encapsulated demenidazole preparation. The particle size range of the encapsulated preparation is 100 μm-200 μm, the recovery rate is 97.9%, the encapsulation rate is 95.2%, and the drug loading is 60.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com