A kind of flaky self-assembled basic copper carbonate curd and its simple preparation method
A self-assembly, copper carbonate technology, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., to achieve the effect of narrow particle size distribution, easily controllable conditions, and difficult to agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
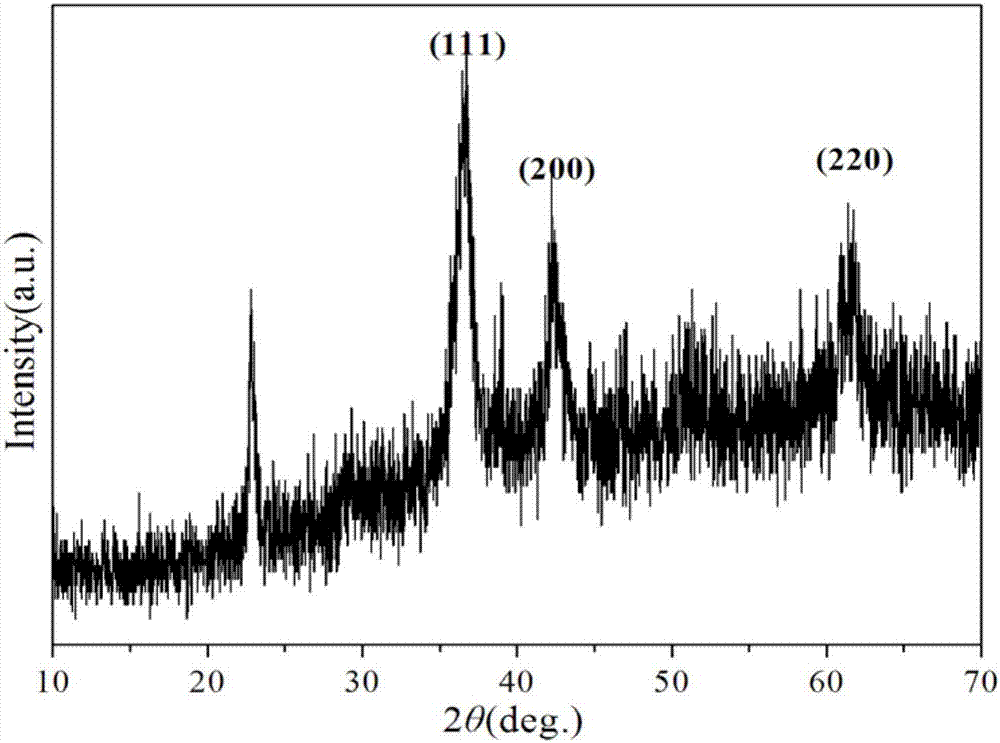
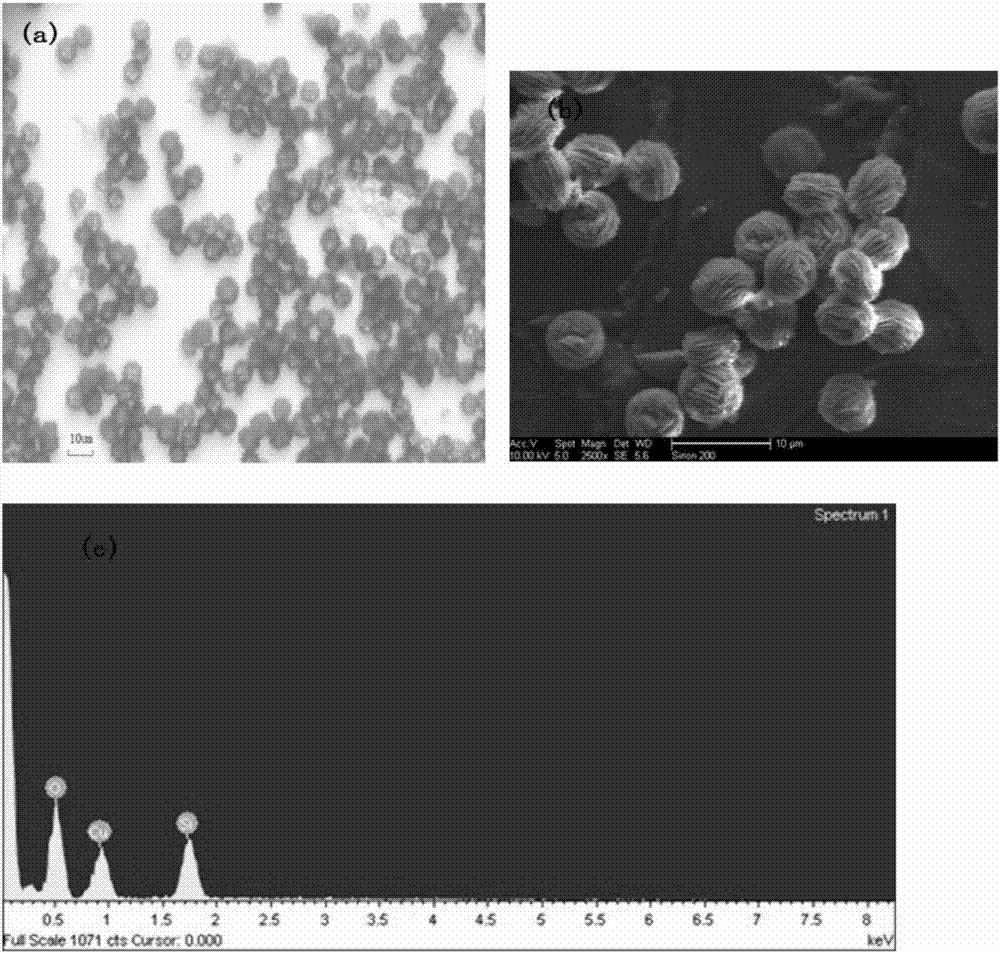
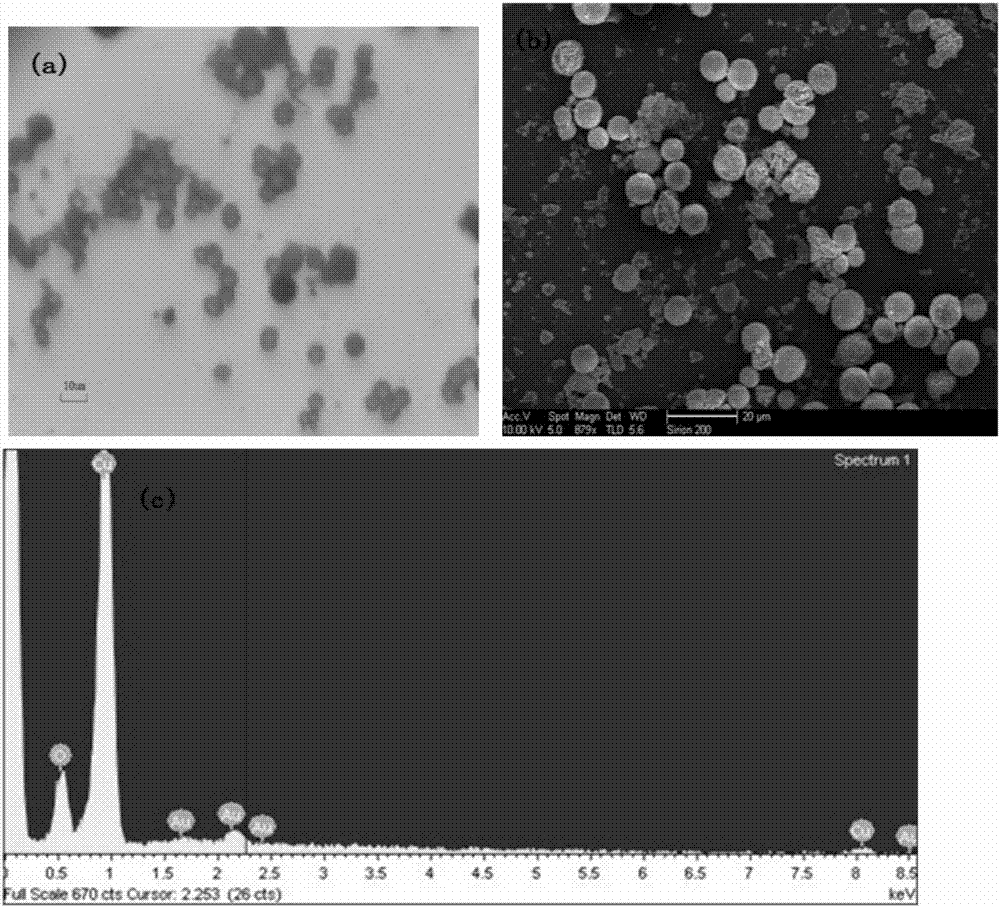
Image
Examples
Embodiment 1
[0024] Preparation of sheet-like self-assembled basic copper carbonate curds:
[0025] (1) Weigh 1 mmol of L-aspartic acid into a beaker, add 10 mL of absolute ethanol and 5 mL of deionized water to dissolve, and stir on a magnetic stirrer for 30 min to form solution A. Weigh 4mmol Cu(NO 3 ) 2 ·3H 2 Pour O into a beaker, add 10 mL of deionized water to dissolve it, keep the same conditions, and stir for 30 min to form solution B. Add the stirred solution A to the solution B and place it in an ultrasonic cleaner for 30 minutes to form a solution C.
[0026] (2) Transfer solution C into the 50mL reaction kettle washed in advance, then rinse the beaker with 5mL deionized water and pour it into the reaction kettle; cover the reaction kettle and put it in a constant temperature blast drying oven, set the temperature to 120°C, react for 6h; after the reaction is over, take out the reaction kettle, centrifuge the powder generated after the reaction with a centrifuge, wash with ab...
Embodiment 2
[0030] Preparation of sheet-like self-assembled basic copper carbonate curds:
[0031] (1) Weigh 2 mmol of L-aspartic acid into a beaker, add 10 mL of absolute ethanol and 5 mL of deionized water to dissolve, and stir on a magnetic stirrer for 30 min to form solution A. Weigh 1 mmol of copper acetate and pour it into a beaker, add 10 mL of deionized water to dissolve it, keep the same conditions, and stir for 30 min to form solution B. Add the stirred solution A to the solution B and place it in an ultrasonic cleaner for 30 minutes to form a solution C.
[0032] (2) Transfer solution C into the 50mL reaction kettle washed in advance, then rinse the beaker with 5mL deionized water and pour it into the reaction kettle; cover the reaction kettle and put it in a constant temperature blast drying oven, set the temperature to 140°C, react for 6h; after the reaction is over, take out the reaction kettle, centrifuge the powder generated after the reaction, wash with absolute ethanol ...
Embodiment 3
[0035] Preparation of sheet-like self-assembled basic copper carbonate curds:
[0036] (1) Weigh 1 mmol of L-aspartic acid into a beaker, add 5 mL of absolute ethanol and 10 mL of deionized water to dissolve, and stir on a magnetic stirrer for 30 min to form solution A. Weigh 1 mmol of copper chloride and pour it into a beaker, add 10 mL of deionized water to dissolve it, keep the same conditions, and stir for 30 min to form solution B. Add the stirred solution A to the solution B and place it in an ultrasonic cleaner for 30 minutes to form a solution C.
[0037] (2) Transfer solution C into the 50mL reaction kettle washed in advance, then rinse the beaker with 5mL deionized water and pour it into the reaction kettle; cover the reaction kettle and put it in a constant temperature blast drying oven, set the temperature to 160°C, react for 10h; after the reaction is over, take out the reactor, centrifuge the powder generated after the reaction, wash with absolute ethanol and de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com