Method for preparing epoxide by halogen-alcohol method
An epoxide and hydroxide technology, applied in the direction of organic chemistry, etc., can solve the problems of intractability, environmental pollution, and high COD in wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A method for preparing epoxides by haloalcohol method, the method may further comprise the steps:
[0081] (1) Haloalcoholization: add water (H 2 O), chlorine, ethylene, and carry out haloalcoholation reaction to obtain chloroethanol; add a catalyst, the catalyst is tungstic acid; the weight ratio of ethylene to the catalyst is 1:0.3.
[0082] (2) saponification: carry out saponification reaction with the chloroethanol of step (1) and sodium hydroxide, separate and obtain oxirane and sodium chloride;
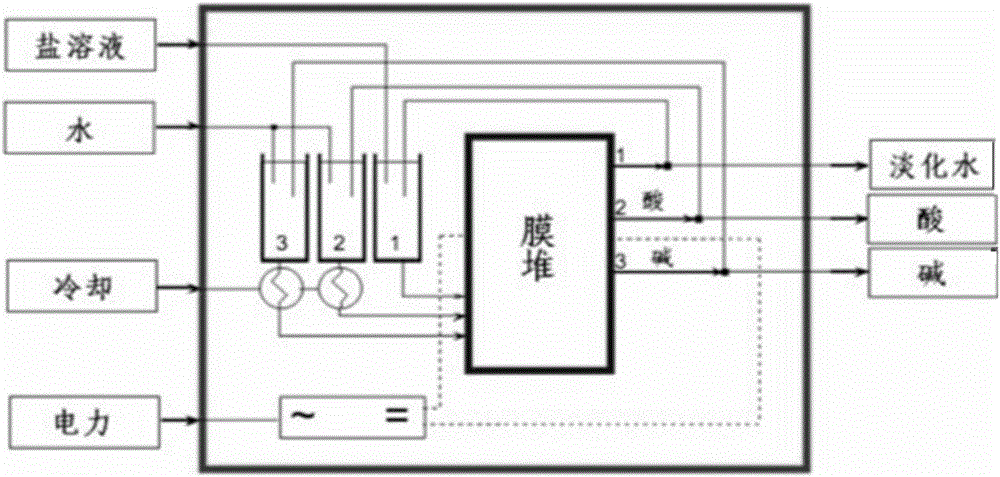
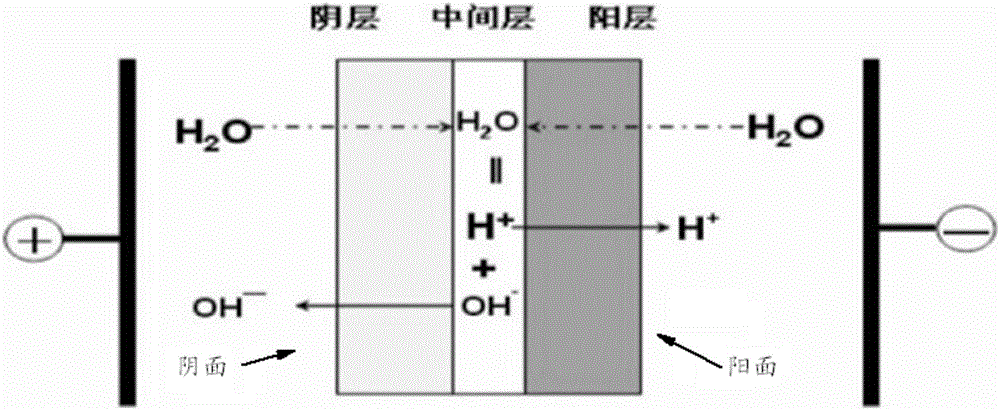
[0083] (3) Electrodialysis: the sodium chloride obtained in step (2) is subjected to bipolar membrane electrodialysis to obtain sodium hydroxide and hydrogen chloride.
[0084] Wherein: the molar ratio of ethylene to chlorine in step (1) is 1:1.5; the molar ratio of ethylene to water is 1:2; the reaction temperature in step (1) is 30°C. The molar ratio of chloroethanol and sodium hydroxide in the step (2) is 1:3; the reaction temperature in the step (2) is 45°C. The re...
Embodiment 2
[0092] A method for preparing epoxides by haloalcohol method, the method may further comprise the steps:
[0093] (1) Halogenation: add H in the reaction device 2 O, chlorine, ethylene, carry out haloalcoholization reaction to obtain chloroethanol; add catalyst, the catalyst is HTS-1 molecular sieve; the weight ratio of ethylene to catalyst is 1:0.5.
[0094] (2) saponification: carry out saponification reaction with the chloroethanol of step (1) and sodium hydroxide, separate and obtain oxirane and sodium halide chloride;
[0095] (3) Electrodialysis: the sodium chloride obtained in step (2) is subjected to bipolar membrane electrodialysis to obtain sodium hydroxide and hydrogen chloride.
[0096] (4) Refining of epoxide, step (2) obtains oxirane to obtain refined oxirane through rectification.
[0097]Wherein: the molar ratio of ethylene and chlorine in step (1) is 1:2; the molar ratio of ethylene and water is 1:5; the reaction temperature in step (1) is 40°C. The molar r...
Embodiment 3
[0099] Repeat embodiment 2, just add the acid binding agent in step (1); The acid binding agent is calcium carbonate. The molar ratio of ethylene to calcium carbonate is 1:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com