Method for analyzing and detecting trace metal ion impurities in dimethyl diallyl ammonium chloride monomers
A technology of dimethyl diallyl ammonium chloride and trace metals, which is applied in the preparation of test samples, measurement of color/spectral properties, etc., can solve the problems of difficulty in ensuring the accuracy and limitation of metal ion content determination results, and the like, Achieve the effect of avoiding operational difficulties and result errors, improving accuracy, and high analytical precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
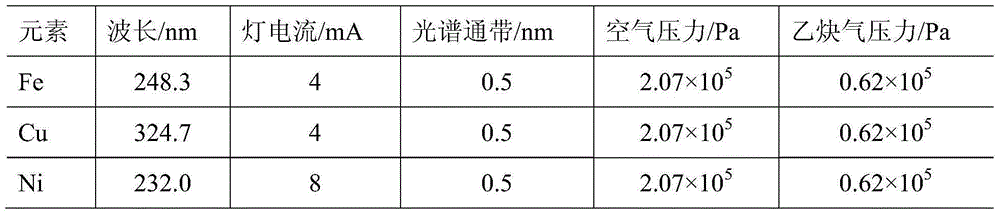
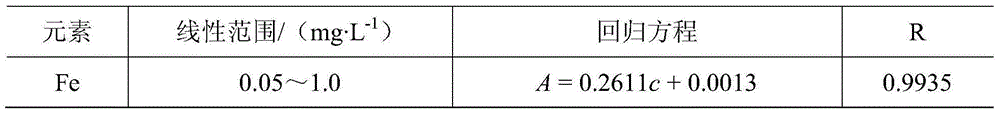
[0035] (1) Preparation of mixed standard working solution
[0036] Accurately pipette certain volumes of Fe, Cu, and Ni single element standard solutions (500 mg / L) respectively, and sequentially add them to high-purity DMDAAC monomer solutions with a mass fraction of 30%, and prepare mass concentrations of 0.05, 0.10, 0.30, 0.50, 0.70, 0.90, 1.00mg / L series of standard working solutions for monomers containing metal ions.
[0037] (2) Complexation reaction
[0038] Take 20mL of different mass concentrations of the metal ion-containing monomer standard working solution in a 50mL separatory funnel, adjust its pH to 1 with nitric acid solution, add 2.0mL complexing agent (the ratio of the amount of substances is 1:1: 1 ammonium pyrrolidinedithiocarbamate, sodium diethyldithiocarbamate and 1,10-phenanthrene phenanthroline), shake and mix to make it fully complexed.
[0039] (3) Extraction and separation
[0040] Use a pipette to accurately pipette 5 mL of methyl isobutyl keton...
Embodiment 2
[0060] (1) Preparation of mixed standard working solution
[0061] Accurately pipette certain volumes of Fe, Cu, and Ni single element standard solutions (500 mg / L) respectively, and sequentially add them to high-purity DMDAAC monomer solutions with a mass fraction of 45%, and prepare mass concentrations of 0.05, 0.10, 0.20, 0.40, 0.60, 0.80, 1.00mg / L series of standard working solutions for monomers containing metal ions.
[0062] (2) Complexation reaction
[0063] Get 20mL of different mass concentrations of the metal ion-containing monomer standard working solution in a 50mL separatory funnel, adjust its pH to 3 with nitric acid solution, add 2.5mL complexing agent (the ratio of the amount of substance is 1:1: 1 ammonium pyrrolidinedithiocarbamate, sodium diethyldithiocarbamate and 1,10-phenanthrene phenanthroline), shake and mix to make it fully complexed.
[0064] (3) Extraction and separation
[0065] Use a pipette to accurately pipette 8 mL of methyl isobutyl ketone ...
Embodiment 3
[0083] (1) Preparation of mixed standard working solution
[0084] Accurately pipette a certain volume of Fe, Cu, Ni single element standard solution (500mg / L) respectively, and sequentially add to the high-purity DMDAAC monomer solution with a mass fraction of 60%, and prepare a mass concentration of 0.05, 0.10, 0.20, 0.40, 0.80, 1.00mg / L series of standard working solutions for monomers containing metal ions.
[0085] (2) Complexation reaction
[0086] Take 20mL of different mass concentrations of the metal ion-containing monomer standard working solution in a 50mL separatory funnel, adjust its pH to 6 with nitric acid solution, add 3.0mL complexing agent (the ratio of the amount of substances is 1:1: 1 ammonium pyrrolidinedithiocarbamate, sodium diethyldithiocarbamate and 1,10-phenanthrene phenanthroline), shake and mix to make it fully complexed.
[0087] (3) Extraction and separation
[0088] Use a pipette to accurately pipette 10 mL of methyl isobutyl ketone into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com