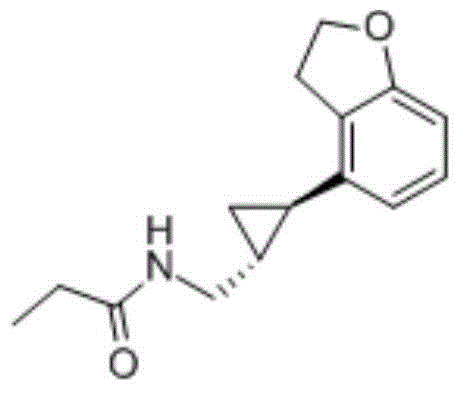
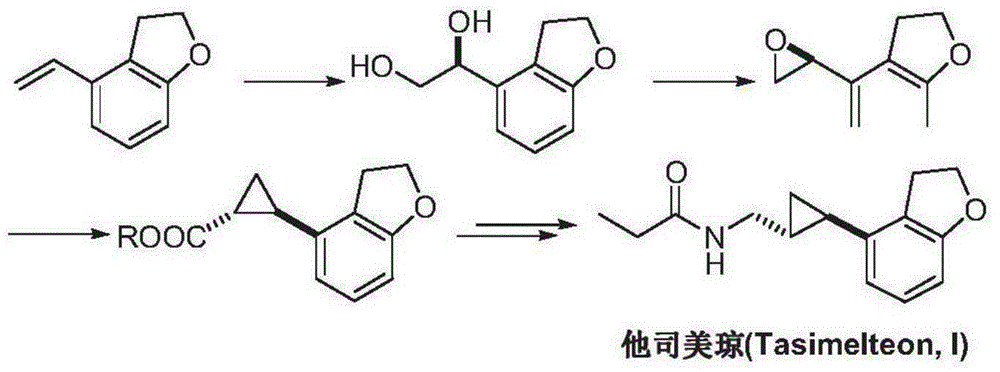
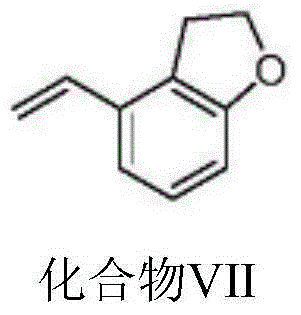
Tasimelteon intermediate and preparation method thereof
A technology of tasimelteon and an intermediate is applied in the field of the intermediate of a drug for treating insomnia and the preparation thereof, and can solve the problems of lack of an intermediate preparation method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of intermediate I
[0037]
[0038] molecular weight Feeding amount Moles / mol equivalent 5,8-Dihydronaphthol (a) 146 2.5kg 17.1 1 Acetonitrile 40 17L potassium carbonate 138 2.36kg 17.1 1 Benzyl bromide 171 2.93kg 17.1 1 ethyl acetate 1L
[0039] Add 2.5kg of raw material a and 15L of acetonitrile into a 20L reactor, stir to dissolve, add 2.36kg of potassium carbonate, add 2.93kg of benzyl bromide under nitrogen protection, and heat up to reflux for 12 hours. After cooling to room temperature, filter with suction, wash the filter cake with 1L ethyl acetate, and concentrate the filtrate to obtain a brown oil. Add 2L of acetonitrile to the concentrate, raise the temperature to reflux and stir for 2h. Cool to room temperature, further cool down to 0°C, continue to stir for 2h, filter with suction, wash the filter cake with 200mL of cold acetonitrile, and blow dry at 35...
Embodiment 2
[0041] Embodiment 2: the synthesis of intermediate II
[0042]
[0043] molecular weight Feeding amount Moles / mol equivalent I 236 2kg 8.5 1 Potassium osmate dihydrate 368 14g 0.038 0.5% potassium carbonate 138 3.48kg 25.2 3 Potassium ferricyanide 329 8.44kg 25.6 3 tert-butanol 20L water 120L Benzyltriethylammonium chloride 100g Methanesulfonamide 95 814g 8.5 1 ethyl acetate 18L
[0044] Add 8.44kg potassium ferricyanide and 20L water into a 100L kettle, stir well, then add 3.48kg potassium carbonate, 14g potassium osmate dihydrate, 20L tert-butanol, 100g benzyltriethylammonium chloride and 814g methanesulfonate Amide, after stirring evenly, add intermediate I and react at 25°C for 16h. Add 40L of water, stir for 2 hours, filter with suction, wash the filter cake with 70L of water until the filtrate is colorless, and dry it by blowing air at 60°C to obtain a g...
Embodiment 3
[0047] Embodiment 3: the synthesis of intermediate II
[0048]
[0049] molecular weight Feeding amount Moles / mol equivalent I 236 2kg 8.5 1 Osmium tetroxide 254.2 10.8g 0.038 0.5% potassium carbonate 138 3.48kg 25.2 3 Potassium ferricyanide 329 8.44kg 25.6 3 tert-butanol 20L water 120L Benzyltriethylammonium chloride 100g Methanesulfonamide 95 814g 8.5 1 ethyl acetate 18L
[0050] Add 8.44kg potassium ferricyanide and 20L water into a 100L kettle, stir well, then add 3.48kg potassium carbonate, 10.8g osmium tetroxide, 20L tert-butanol, 100g benzyltriethylammonium chloride and 814g methanesulfonamide After stirring evenly, intermediate I was added and reacted at 25°C for 12h. Add 40L of water, stir for 0.5h, filter with suction, wash the filter cake with 70L of water until the filtrate is colorless, and dry it under air blast at 60°C to obtain a gray solid.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com