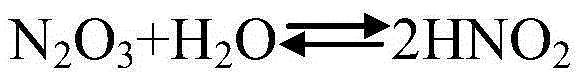Methyl nitrite preparation method by reaction of NO and nitric acid and methanol
A technology of methyl nitrite and methanol, applied in the preparation of nitrite, organic chemistry and other directions, can solve the problems of equipment corrosion and high nitrogen oxide supply cost, and achieve the effect of good technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
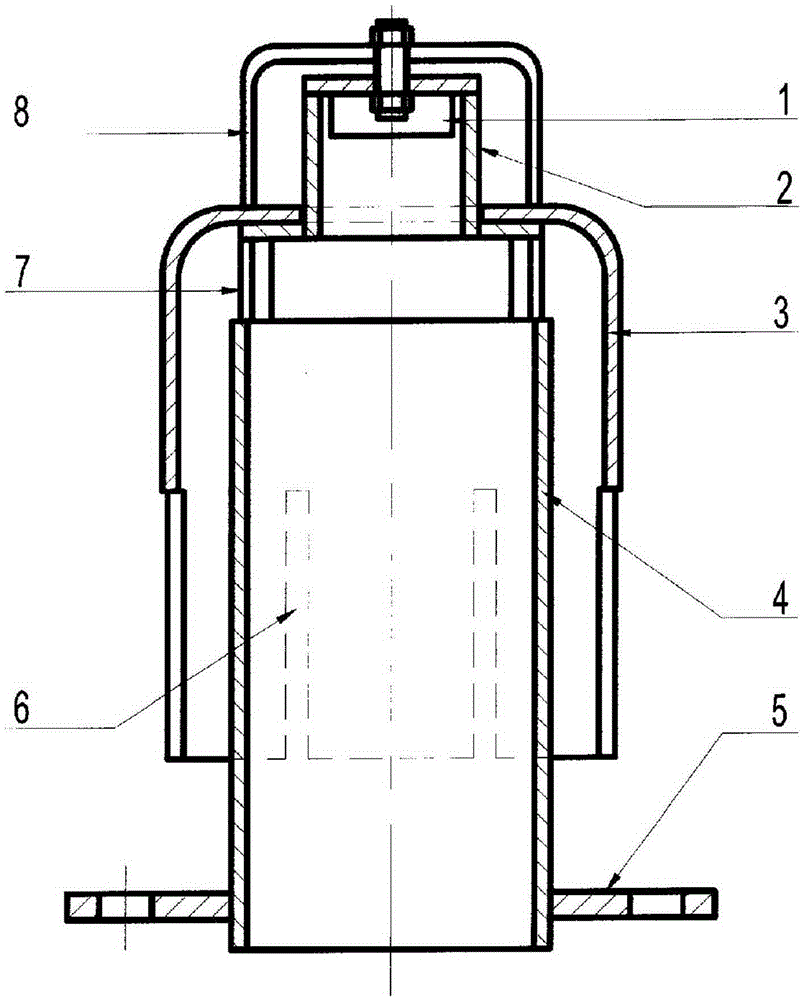
[0040] The configuration has figure 1 Trickle bed reactor with gas-liquid distributor shown. Wherein, the diameter of the top of the bubble cap of the gas-liquid distributor is equal to the diameter of the bottom thereof, and four rectangular slits are uniformly arranged on the lower part of the bubble cap. The catalyst in the trickle bed reactor is supported by activated carbon, and the catalyst is composed of: 15% nickel metal, 2% iron as a metal additive, 3% titanium as a metal additive, and the balance as a carrier. The mixed liquid (nitric acid: 1%wt, methanol: 99%wt) and the mixed gas (CO: 25%v / v, NO: 10%v / v, CO 2 : 15% v / v, MN: 5% v / v, N 2 : 45% v / v) into the trickle bed reactor respectively, the reaction temperature is 70°C, the reaction pressure is normal pressure, and the liquid hourly space velocity is 0.5h -1 , NO and nitric acid molar ratio of 8 reacted to generate methyl nitrite, the conversion rate of nitric acid was 95.1%.
Embodiment 2
[0042] The configuration has figure 1 Trickle bed reactor with gas-liquid distributor shown. Wherein, the top diameter of the bubble cap of the gas-liquid distributor is smaller than the bottom diameter, and the lower part of the bubble cap is uniformly provided with four slits in the shape of isosceles triangles. The catalyst in the trickle bed reactor uses silicon oxide as a carrier, and the catalyst composition is: 5% metallic nickel, 10% titanium as a metal additive, and the rest as carrier. The mixed liquid (nitric acid: 15%wt, water: 30%wt, methanol: 55%wt) and the mixed gas containing NO (CO: 30%v / v, NO: 15%v / v, CO 2 : 15% v / v, MN: 5% v / v, N 2 : 35% v / v) into the trickle bed reactor respectively, the reaction temperature is 75°C, the reaction pressure is 0.35MPa, and the liquid hourly space velocity is 6h -1 , NO and nitric acid molar ratio of 3 reacted to generate methyl nitrite, the conversion rate of nitric acid was 95.6%.
Embodiment 3
[0044] The configuration has figure 1 Trickle bed reactor with gas-liquid distributor shown. Wherein, the diameter of the top of the bubble cap of the gas-liquid distributor is equal to the diameter of the bottom, and the lower part of the bubble cap is evenly provided with 8 rectangular slits. The catalyst in the trickle bed reactor is supported by activated carbon, and the catalyst is composed of: 25% nickel metal, 1% iron as a metal additive, titanium as a metal additive, and the rest as a carrier. The mixed liquid (nitric acid: 5%wt, water: 8%wt, methanol: 87%wt) and the mixed gas containing NO (CO: 20%v / v, NO: 15%v / v, CO 2 : 10% v / v, MN: 3% v / v, N 2 : 52% v / v) into the trickle bed reactor respectively, the reaction temperature is 80 ℃, the reaction pressure is 0.6MPa, and the liquid hourly space velocity is 8h -1 , the molar ratio of NO to nitric acid was 2.5 to react to generate methyl nitrite, and the conversion rate of nitric acid was 96.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com