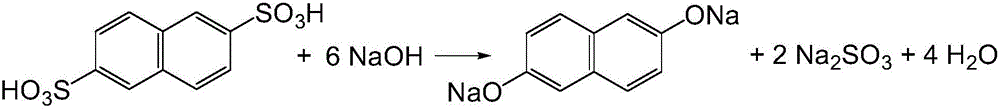
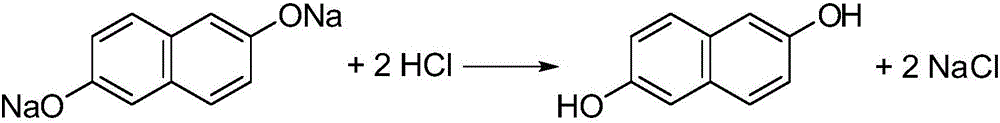
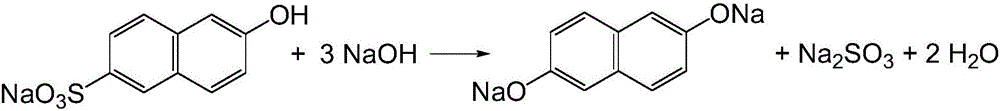
Preparation method of 2,6-dihydroxynaphthalene
A technology of dihydroxynaphthalene and hydroxyl, which is applied in the field of preparation of 2,6-dihydroxynaphthalene, can solve the problems of unsuitability for industrial production, many oxidation by-products, and low product quality, and achieve a stable and safe reaction process and reduce tar generation , The effect of simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Add 200g of hydrogenated terphenyl and 50g of water into a 500ml four-necked bottle, slowly add 50.3g of 2,6-naphthalene disulfonic acid with a content of 80.11% (calculated as sulfuric acid, total acid content is 31.57%) while stirring, and stir Evenly, then slowly add 13.5g of 96% sodium hydroxide to neutralize 2,6-naphthalene disulfonic acid until neutral.
[0052] (2) Transfer the above-mentioned materials from the four-necked bottle to a stainless steel reaction kettle connected with a vacuum dehydration device, add 46.7g96% sodium hydroxide, 44.0g85% potassium hydroxide and 0.2g2,6-di-tert-butyl For p-cresol, close the reaction kettle, replace the air in the kettle with nitrogen three times, then start stirring, turn on the vacuum pump to -0.02MPa, slowly open the steaming valve of the reaction kettle to maintain the pressure in the kettle at -0.02MPa, heat up and distill water. When the temperature reaches 110°C, the water starts to flow out, and when it reac...
Embodiment 2
[0055] (1) Add 160g of hydrogenated terphenyl and 40g of water into a 500ml four-neck bottle, slowly add 39.4g of 2-hydroxy-6-naphthalenesulfonic acid sodium salt with a content of 87.39% while stirring, stir evenly, and then add 5.8g of 96% hydrogen Sodium oxide neutralizes the sodium salt of 2-hydroxy-6-naphthalenesulfonate to neutrality.
[0056] (2) Transfer the above material from the four-necked bottle to a stainless steel reaction kettle connected with a vacuum dehydration device, add 28.0g96% sodium hydroxide, 36.9g85% potassium hydroxide and 0.2g tetrakis (3,5-di tert-butyl-4-hydroxyhydrocinnamic acid) pentaerythritol ester, close the reactor, replace the air in the reactor with nitrogen for three times, then start stirring, turn on the vacuum pump to -0.02MPa, slowly open the steam valve of the reactor to maintain the pressure in the reactor as - 0.02MPa, heated up distilled water. When the temperature reaches 153°C, the water starts to flow out, and when it reaches...
Embodiment 3
[0059] (1) Add 140.2g of hydrogenated terphenyls recovered from the previous batch reaction, 9.8g of new hydrogenated terphenyls and 40g of water into a 500ml four-necked bottle, and slowly add 39.4g of 2-hydroxy-6-naphthalene with a content of 87.39% while stirring Sodium sulfonic acid, stir evenly, then add 8.2g of 85% potassium hydroxide to neutralize the sodium salt of 2-hydroxy-6-naphthalenesulfonic acid until neutral.
[0060] (2) Transfer the above-mentioned materials from the four-necked bottle to a stainless steel reaction kettle connected with a vacuum dehydration device, add 31.1g96% sodium hydroxide, 21.5g85% potassium hydroxide and 0.1g tetrakis (3,5-di Tert-butyl-4-hydroxyhydrocinnamic acid) pentaerythritol ester, close the reactor, replace the air in the reactor with nitrogen for three times, then start stirring, turn on the vacuum pump to -0.03MPa, slowly open the steam valve of the reactor to maintain the pressure in the reactor as - 0.03MPa, heated up distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com