Synthetic method for cyclopropyl diphenyl sulfonium trifluoromethanesulfonic salt
A technology of cyclopropyl diphenyl sulfonium and trifluoromethanesulfonate is applied in the synthesis field of sulfur ylide reagent, can solve the problems of complex synthesis process, difficult to be widely used and the like, and achieves high yield, easy operation and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
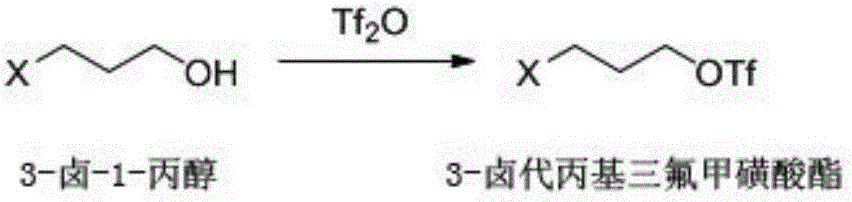
[0030] (a) Synthesis of 3-bromopropyl trifluoromethanesulfonate: 13.9g (0.10mol) 3-bromopropanol, 8.7g (0.11mol) pyridine and 150mL dichloromethane were added in a 250mL three-necked flask, cooled to 0°C. Slowly add 29.63 g (0.105 mol) of trifluoromethanesulfonic anhydride dropwise under the conditions of stirring and temperature at -20°C. After the dropwise addition, the temperature is naturally raised to room temperature and reacted overnight. After the reaction finishes, add 30mL concentration to the reaction solution and be the dilute hydrochloric acid of 0.1mol / L, stir and separate layers, extract the organic phase, then wash the organic phase with 0.1mol / L sodium carbonate aqueous solution and saturated sodium chloride aqueous solution respectively, The organic phase was dried with anhydrous sodium sulfate and concentrated to obtain 22 g of 3-bromopropyl triflate with a yield of 81%.
[0031] The reaction formula is as follows:
[0032]
[0033] (b) Synthesis of 3-b...
Embodiment 2
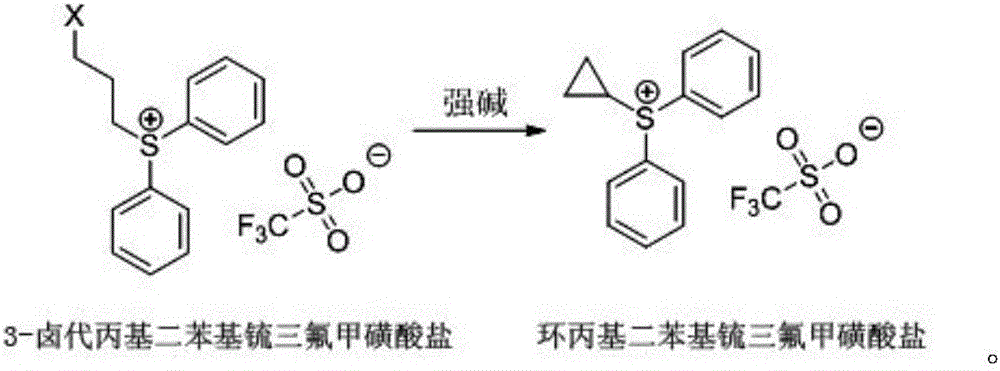
[0041] Cyclopropyldiphenylsulfonium trifluoromethanesulfonate was synthesized according to the following steps, and the synthetic route was the same as in Example 1.
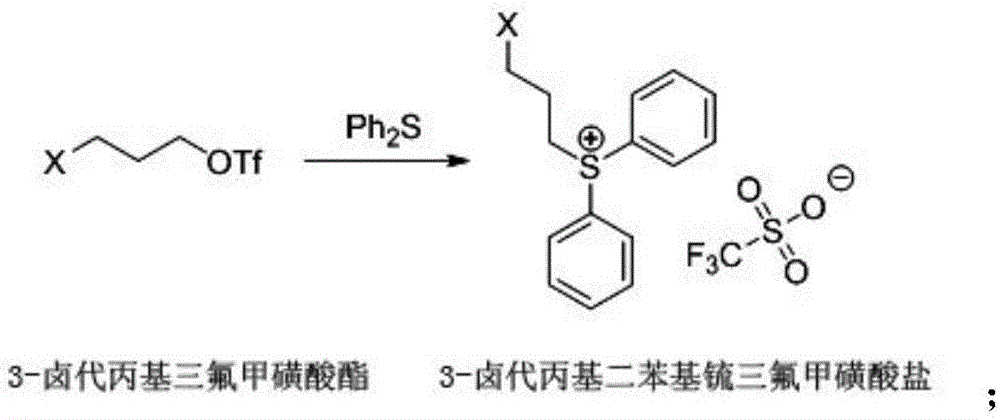
[0042] (a) Synthesis of 3-chloropropyl trifluoromethanesulfonate: 9.5g (0.10mol) 3-chloropropanol, 11.2g (0.11mol) triethylamine and 100mL chloroform were added in a 250mL three-necked flask, cooled to 0°C. 31.2 g (0.11 mol) of trifluoromethanesulfonic anhydride was slowly added dropwise at a temperature of -10° C. After the dropwise addition, the temperature was naturally raised to room temperature and reacted overnight. After the reaction finishes, add 30mL concentration to the reaction solution and be the dilute hydrochloric acid of 0.1mol / L, stir and separate layers, extract the organic phase, then wash the organic phase with 0.1mol / L sodium carbonate aqueous solution and saturated sodium chloride aqueous solution respectively, The organic phase was dried with anhydrous sodium sulfate and concentrated to ob...
Embodiment 3
[0046] Cyclopropyldiphenylsulfonium trifluoromethanesulfonate was synthesized according to the following steps, and the synthetic route was the same as in Example 1.
[0047] (a) Synthesis of 3-iodopropyl trifluoromethanesulfonate: 25g (0.134mol) 3-iodopropanol, 26g (0.202mol) diisopropylethylamine and 150mL 1,2-dichloroethyl Thane was added into a 250mL three-necked flask, and the temperature was lowered to 0°C. 56.9 g (0.202 mol) of trifluoromethanesulfonic anhydride was slowly added dropwise under stirring at a temperature of 0° C. After the dropwise addition, the temperature was naturally raised to room temperature and reacted overnight. After the reaction finishes, add 30mL concentration to the reaction solution and be the dilute hydrochloric acid of 0.1mol / L, stir layering, extract the organic phase, then wash the organic phase with 0.1mol / L sodium carbonate aqueous solution and saturated sodium chloride aqueous solution respectively, The organic phase was dried with an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com