Green oxidation bifunctional catalyst, preparation method and applications thereof
A dual-function catalyst and catalyst technology, which is applied in the field of catalysis, can solve problems such as the inability to solve the problem of engineering implementation of raw materials and prices of supported gold catalysts in the whole process of dark operation, and achieve the effects of green preparation, oxidation inhibition, and high-efficiency preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
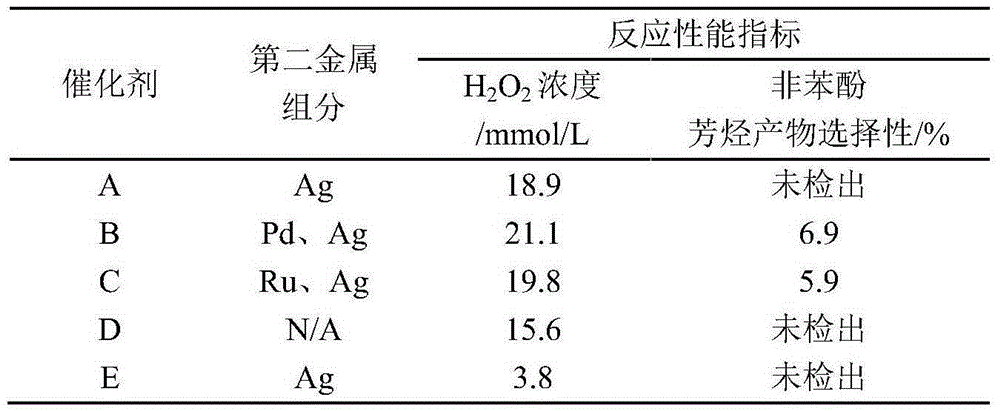
[0023] Take 0.755g Cu(NO 3 ) 2· 9H 2 O with 0.315g AgNO 3 Dissolve in 30ml of deionized water to prepare a solution I with a metal ion concentration of 0.170mol / L; weigh 0.612g of NaBH in an ice-water bath 4 Dissolve 0.064g NaOH in 16ml deionized water, add 4g TS-1 molecular sieve carrier with a silicon-titanium molar ratio of 50, and continue stirring for 0.5h to obtain 1.0mol / L NaBH 4 Solution II of the reducing agent.
[0024] Keep the ice-water bath and stirring conditions, use a peristaltic pump to slowly add solution I to solution II, continue stirring for 0.5h after the addition is completed, and slowly add 0.5mol / L HCl aqueous solution to the excess NaBH 4 Completely decompose, showing that there are no more bubbles; after continuing to stir for 0.5h, high-speed centrifugation, and washing with deionized water until no chlorine ions are detected in the clear solution on the upper layer of the centrifuge tube, the solid is moved to a vacuum drying oven, and it is re...
Embodiment 2
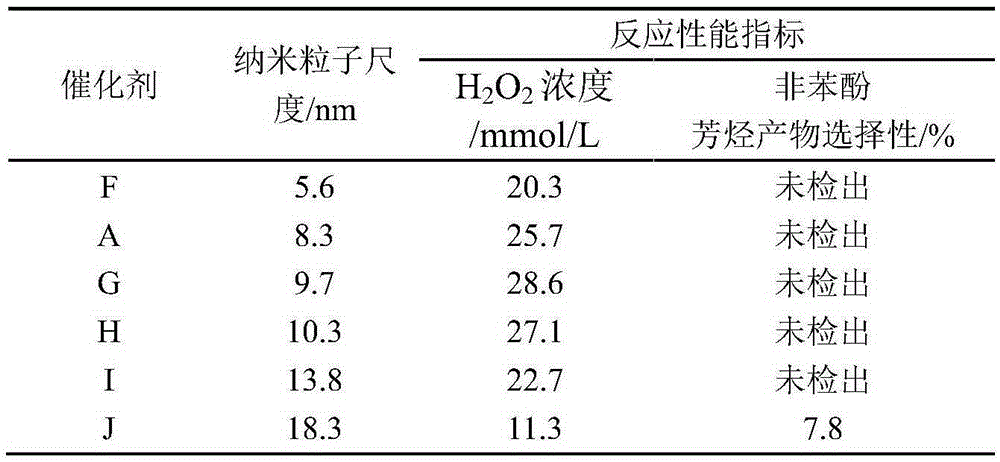
[0026] Take 0.755g Cu(NO 3 ) 2· 9H 2 O with 0.315g AgNO 3 and 0.334g PdCl 2 Dissolve in 30ml of deionized water to prepare a solution I with a metal ion concentration of 0.220mol / L; weigh 0.871g of NaBH in an ice-water bath 4 Dissolve 0.092g NaOH in 23ml of deionized water, add 4g of TS-1 molecular sieve carrier with a silicon-titanium molar ratio of 50, and continue stirring for 0.5h to obtain 1.0mol / L NaBH 4 Solution II of the reducing agent.
[0027] Keep the ice-water bath and stirring conditions, use a peristaltic pump to slowly add solution I to solution II, continue stirring for 0.5h after the addition is completed, and slowly add 0.5mol / L HCl aqueous solution to the excess NaBH 4 Completely decompose, showing that there are no more bubbles; after continuing to stir for 0.5h, high-speed centrifugation, and washing with deionized water until no chlorine ions are detected in the clear solution on the upper layer of the centrifuge tube, the solid is moved to a vacuum ...
Embodiment 3
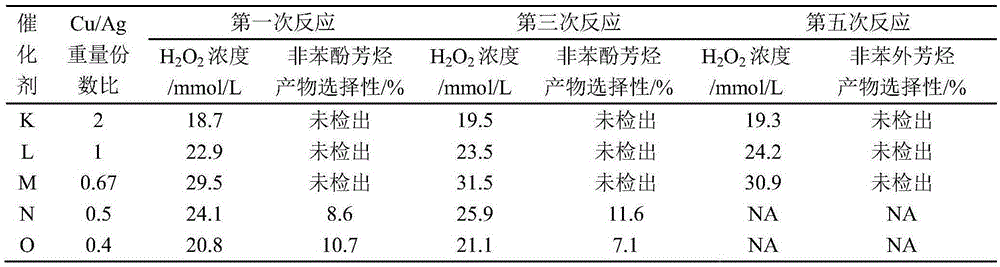
[0029] Take 0.755g Cu(NO 3 ) 2· 9H 2 O with .315g AgNO 3 and 0.506g RuCl 3· 3H 2 O was dissolved in 30ml deionized water to prepare a solution I with a metal ion concentration of 0.220mol / L; under ice-water bath conditions, weigh 1.003g NaBH 4 Dissolve 0.108g of NaOH in 27ml of deionized water, add 4g of TS-1 molecular sieve carrier with a silicon-titanium molar ratio of 50, and continue stirring for 0.5h to obtain 1.0mol / L NaBH 4 Solution II of the reducing agent.
[0030] Keep the ice-water bath and stirring conditions, use a peristaltic pump to slowly add solution I to solution II, continue stirring for 0.5h after the addition is completed, and slowly add 0.5mol / L HCl aqueous solution to the excess NaBH 4 Completely decompose, showing that there are no more bubbles; after continuing to stir for 0.5h, high-speed centrifugation, and washing with deionized water until no chlorine ions are detected in the clear solution on the upper layer of the centrifuge tube, the solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com