Preparation method of dry suspension granules of cefixime
A cefixime and dry suspension technology, applied in the field of medicine, can solve the problems of poor solubility and unstable cefixime preparation, and achieve the effects of improving stability and solubility, improving acceptability and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] According to the above formula, the present invention also provides a preparation method of cefixime dry suspension particles, wherein, by weight percentage, comprising:
[0037] Step A, get 5-20% of cefixime, 1-10% of disintegrant, 30-70% of sweetener a and 1-10% of suspending agent after sieving respectively, after fully mixing, add wetting agent to make. The soft material is sieved and then dried to obtain cefixime dry suspension intermediate particles.
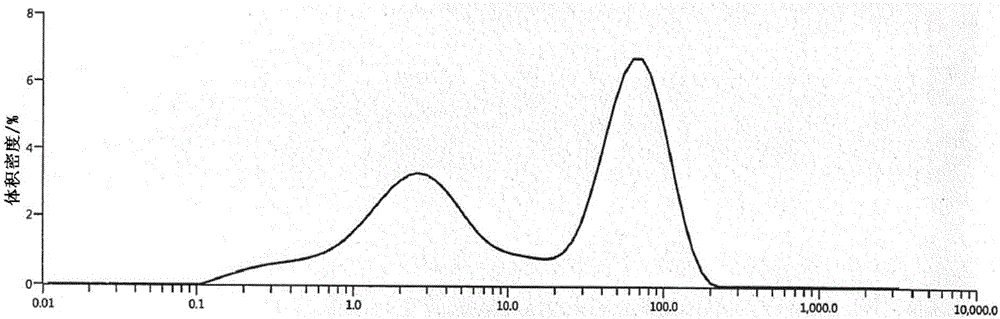
[0038] The particle size distribution diagram of cefixime of the present invention is as follows figure 1 shown, wherein, Dv(10)100um. Where Dv (Volume Density) is the volume density.
[0039] Preferably, the disintegrant can be starch, pregelatinized starch, sodium carboxymethyl starch, hydroxypropyl starch, dextrin, croscarmellose sodium, croscarmellose calcium low-substituted One or more of hydroxypropyl cellulose, cross-linked polyvinyl pyrrolidone, etc.
[0040] Preferably, the sweetener a can be sucrose, f...
Embodiment 1
[0058] The preparation process is as follows:
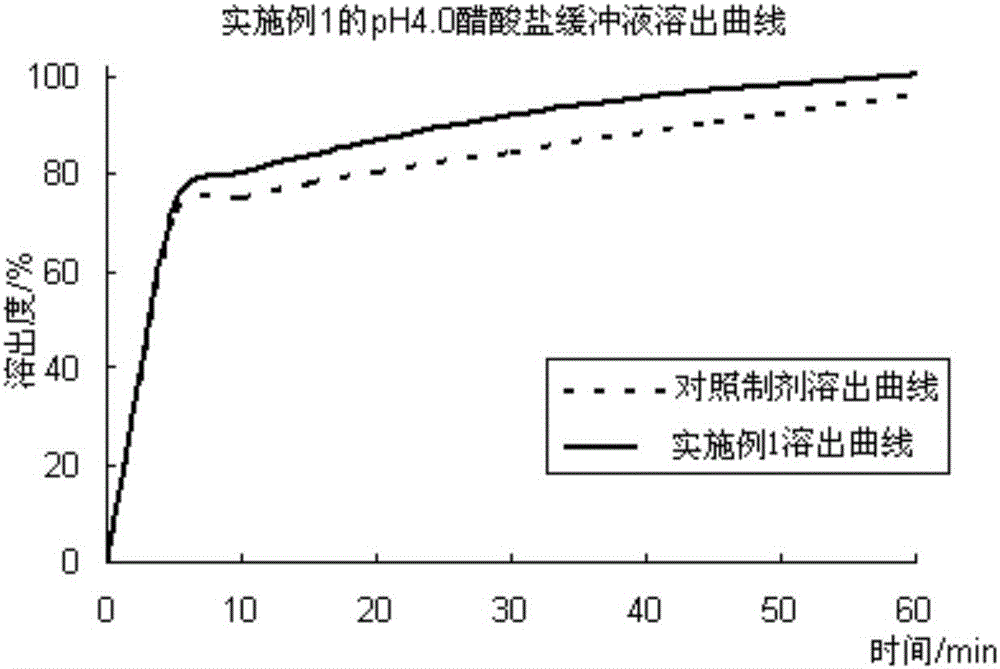
[0059] The first step: under the condition of 23 ℃ / 55% RH, take cefixime, disintegrating agent, sweetener a, and suspending agent to pass through an 80-mesh sieve, put them in a multi-directional mixer and mix for 15 minutes, add 50 % ethanol is used as a wetting agent, and it is made into a soft material and sieved through a 30-mesh sieve, and then dried in a fluidized bed at 50 ° C to obtain cefixime dry suspension intermediate granules; the second step is to take the sweetener b. , colorant and hydroxypropyl methylcellulose are added to 50% ethanol solution, fully dissolved to obtain auxiliary material solution, add pH adjuster to adjust pH value to 3.0, then slowly spray on the surface of cefixime dry suspension intermediate particles, After drying at 40°C, the solubilizer and the flavoring agent are finally added and mixed in a multidirectional mixer for 15 minutes to obtain cefixime dry suspension granules. In the preparat...
Embodiment 2
[0069] The preparation process is as follows:
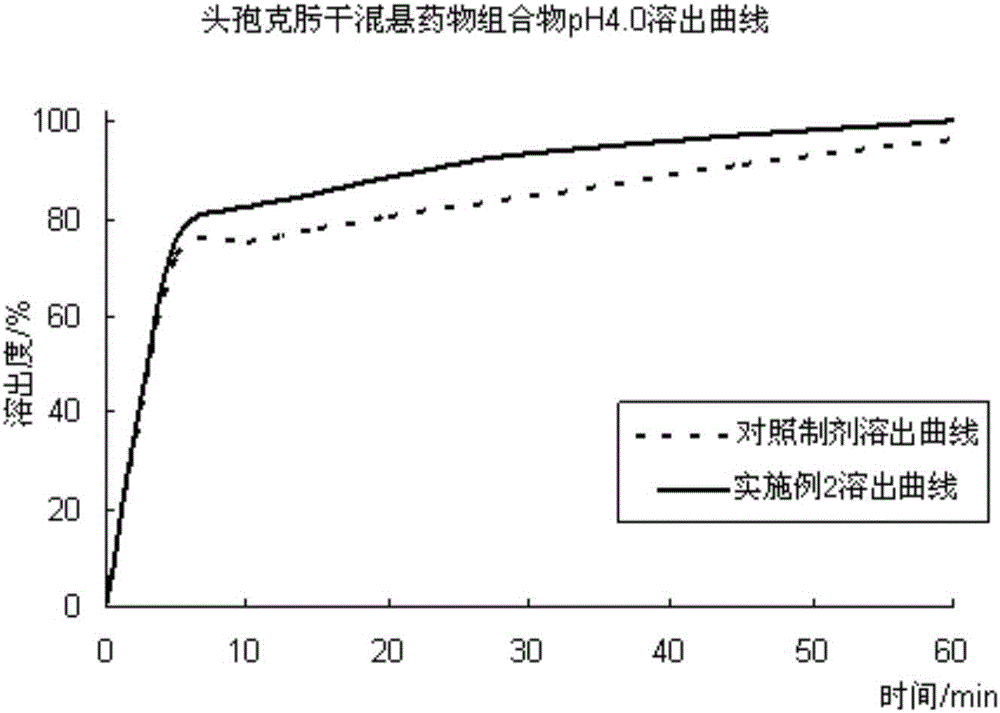
[0070] The first step: under the condition of 22 ℃ / 60% RH, take cefixime, disintegrating agent, sweetener a and suspending agent and pass through 80 mesh sieves, put them in a multidirectional mixer and mix for 25min, add 50 % ethanol is used as a wetting agent, and it is made into a soft material and passed through a 30-mesh sieve, and then dried in a fluidized bed at 45 ° C to obtain cefixime dry suspension intermediate granules; the second step is to take the sweetener b , colorant and hydroxypropyl methylcellulose are added to 30% ethanol solution, fully dissolved to obtain auxiliary material solution, add pH adjuster to adjust pH value to 3.5, then slowly spray on the surface of cefixime dry suspension intermediate particles, Drying treatment at 40°C, finally adding solubilizer and flavoring agent and mixing in a multidirectional mixer for 20 minutes to obtain cefixime dry suspension granules. In the preparation process, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com