Duloxetine hydrochloride enteric-coated tablets and preparation method thereof
A loxetine enteric and hydrochloric acid technology, applied in the direction of pharmaceutical formulation, coating, pill delivery, etc., can solve the problems of slow onset of action, instability to light, instability, etc., to reduce the fluctuation of blood drug concentration, improve The effect of bioavailability and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
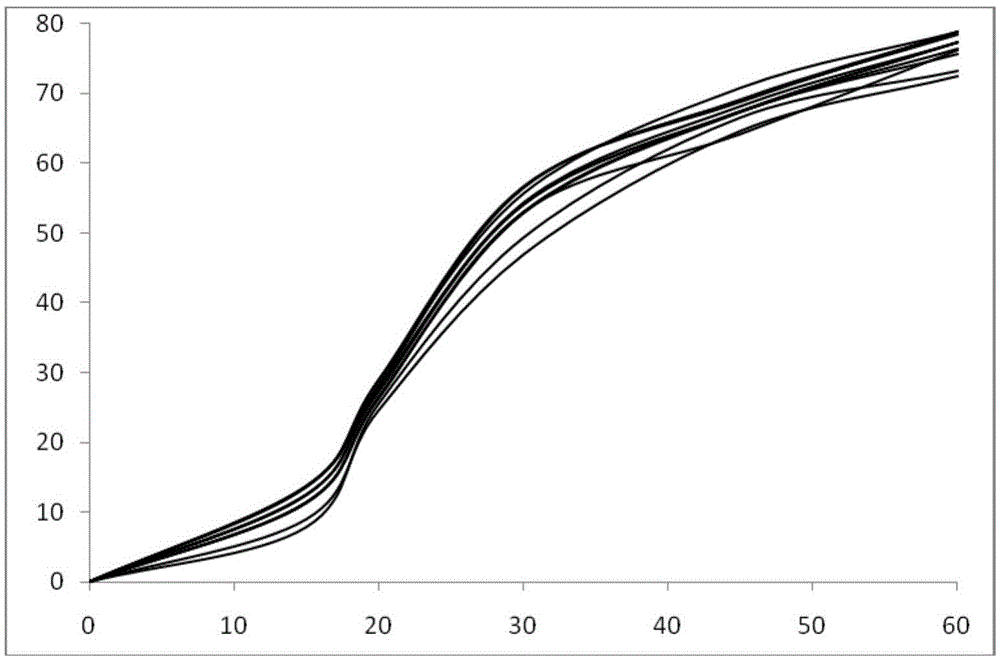
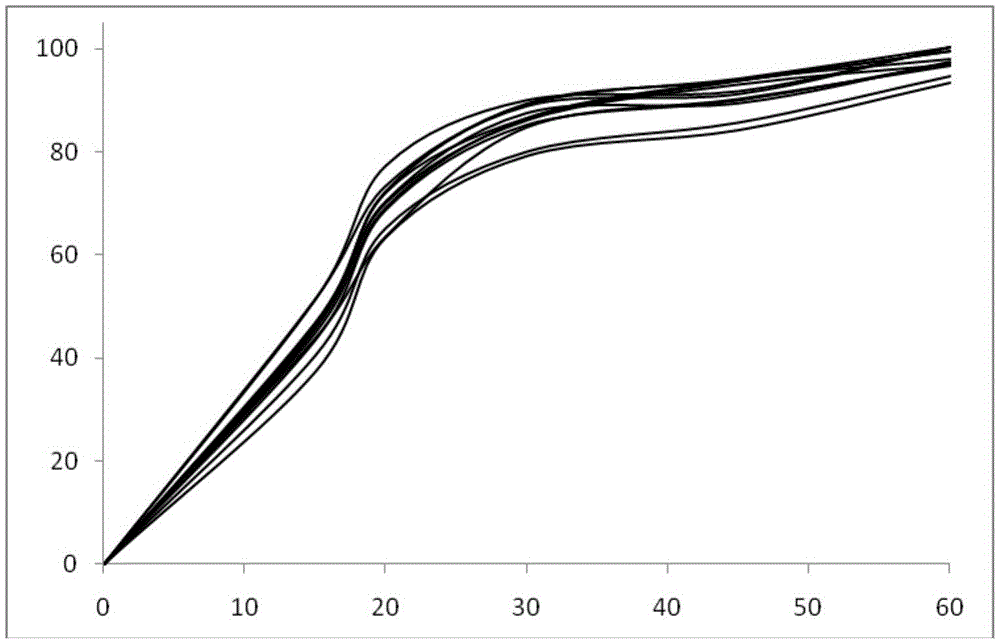
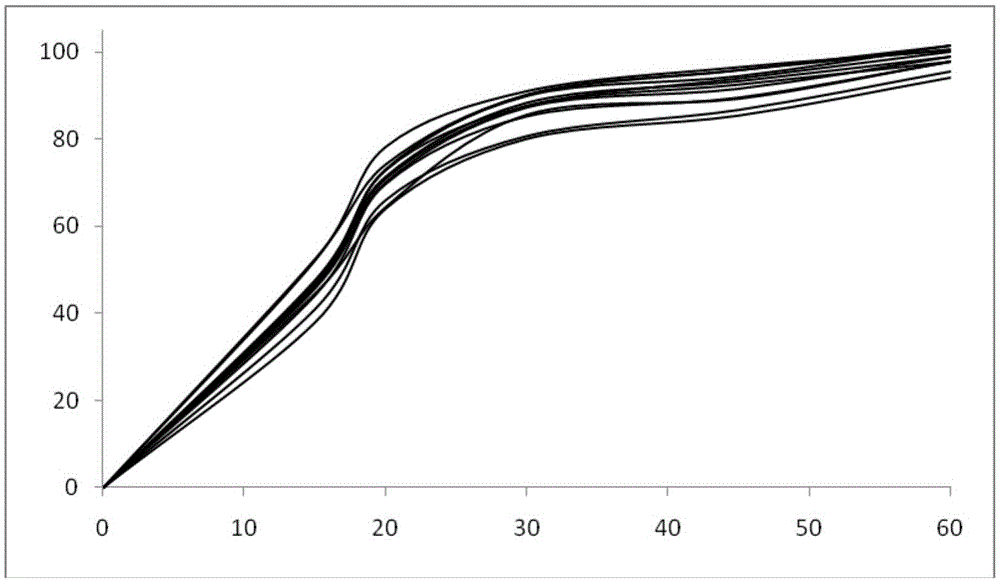
Image
Examples
Embodiment 1
[0034] Example 1 Duloxetine Hydrochloride Enteric-coated Tablets:
[0035]
[0036] The specification of duloxetine hydrochloride enteric-coated tablet of the present invention is:
[0037] 20mg based on duloxetine.
[0038] Another object of the present invention provides the preparation method of described a kind of duloxetine hydrochloride enteric-coated tablet, and this method comprises the following steps:
[0039] (1) Crushing of raw and auxiliary materials: mixing the raw and auxiliary materials of duloxetine hydrochloride, starch, and microcrystalline cellulose into 100-mesh pulverization;
[0040] (2) Adhesive preparation: Weigh 0.315kg pregelatinized starch, slowly add freshly boiled purified water (2.835kg) cooled to room temperature under stirring, stir to dissolve, and make 10% pregelatinized starch pulp spare;
[0041] (3) Granulation: Add the crushed raw and auxiliary materials into the high-speed mixing granulator, close the body, turn on low-speed stirri...
Embodiment 2
[0053] Example 2 Duloxetine Hydrochloride Enteric-Coated Tablets:
[0054]
[0055] The specification of duloxetine hydrochloride enteric-coated tablet of the present invention is:
[0056] 60mg based on duloxetine.
[0057] The preparation method comprises the following steps:
[0058] (1) Crushing of raw and auxiliary materials: mixing the raw and auxiliary materials of duloxetine hydrochloride, starch, and microcrystalline cellulose into 100-mesh pulverization;
[0059] (2) Adhesive preparation: Weigh 0.33kg pregelatinized starch, slowly add freshly boiled purified water (2.97kg) cooled to room temperature under stirring, stir to dissolve, and make 10% pregelatinized starch pulp spare;
[0060] (3) Granulation: Add the crushed raw and auxiliary materials into the high-speed mixing granulator, close the body, turn on low-speed stirring and mixing for 6 minutes to make the material uniform, add pregelatinized starch slurry, turn on low-speed stirring (3 minutes) and the...
Embodiment 3
[0071] Embodiment 3 The research design test of the present invention:
[0072] (1) Formula screening
[0073] Quality characteristics of raw materials:
[0074] API contains C 18 h 19 NOS·HCl shall not be less than 98.5% (calculated on dry basis). Duloxetine hydrochloride is white or off-white crystalline powder, almost non-hygroscopic; this product is easily soluble in methanol, chloroform or glacial acetic acid, soluble in acetonitrile, slightly soluble in water, and soluble in ethyl acetate or hydrochloric acid solution Very slightly soluble, almost insoluble in sodium hydroxide solution.
[0075] Compatibility of APIs and excipients:
[0076] (1) Tablet core accessories
[0077] The basis for the selection of excipients
[0078] The commonly used excipients of the tablet are optimally combined, and the formula composition is determined as the main ingredient, starch, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, and magnesium stearate. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com