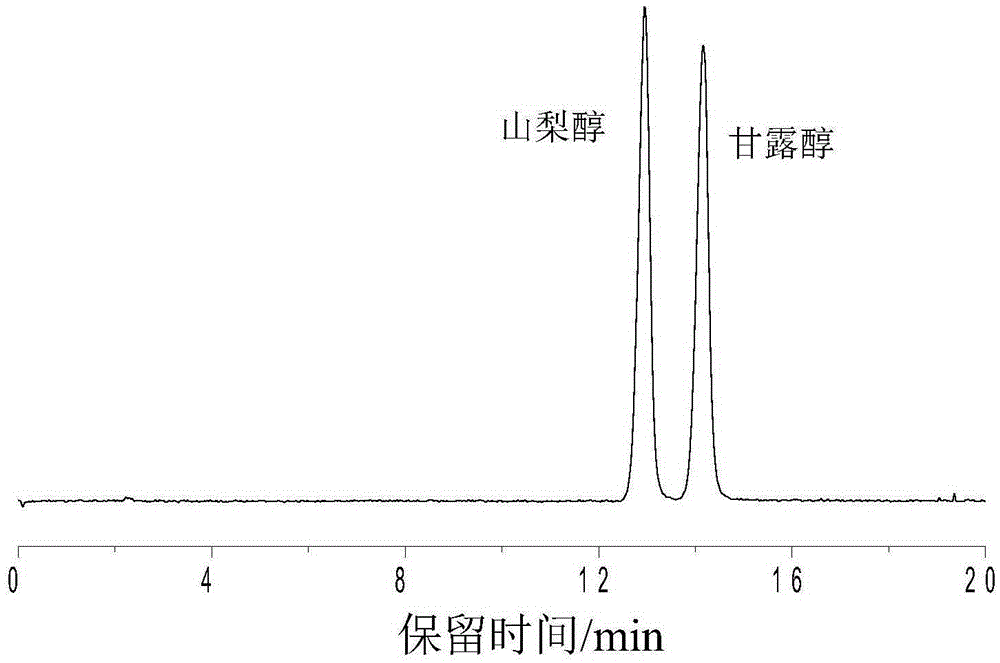
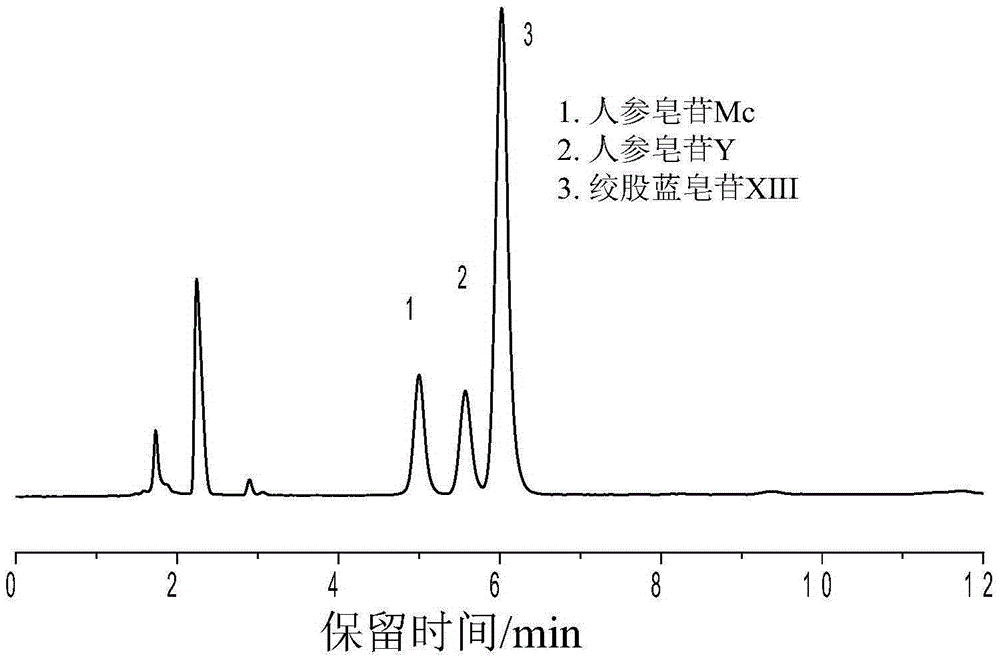
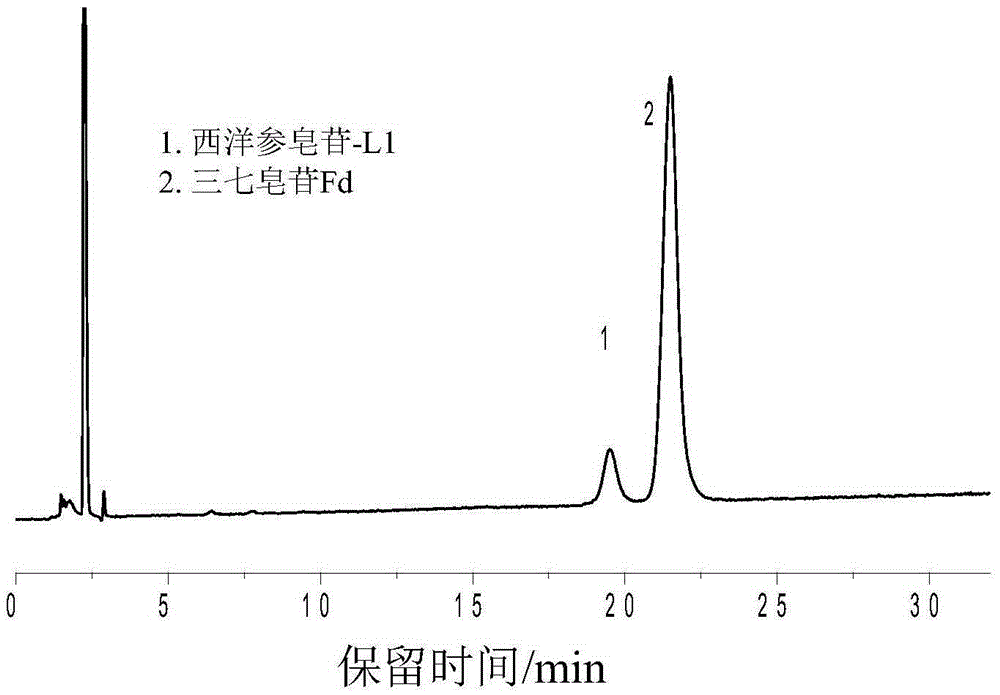
Sulfydryl Michael addition reaction-based silica gel matrix chromatography separation material and preparation thereof
A silica gel-based, chromatographic separation technology, applied in the field of silica gel-based chromatographic separation materials, achieves the effects of novel structure, wide application range, and simple and reliable preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 20g spherical mesoporous silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 300m 2 / g) In a 500 mL glass flask, add 200 mL of 10% hydrochloric acid solution by volume, reflux at 110° C. for 24 hours, and cool to room temperature. Filter, wash with water until neutral, and dry at 150°C for 24 hours. Under a nitrogen atmosphere, 150 mL of anhydrous toluene was added to dry silica gel, and after stirring evenly, 10 mL of trimethoxymercaptopropylsilane and 4 mL of pyridine were added, and stirred at reflux at 110°C for 24 hours. After the reaction system was cooled to room temperature, it was washed with toluene, methanol, water, tetrahydrofuran and methanol in sequence, and dried at 80° C. for 12 hours to obtain mercapto silica gel. Under a nitrogen atmosphere, take 13.5 g of [3-(3-acrylamidopropyl) dimethylamine] propanesulfonic acid and place it in a 150 mL three-necked round bottom flask, add 45 mL of water, and stir until the solid is ...
Embodiment 2
[0034] Weigh 10g of spherical mesoporous silica gel (particle size is 5 μm, pore size is 30nm, specific surface area is 75m2 / g) In a 250 mL glass flask, add 100 mL of 10% nitric acid solution by volume, reflux at 100° C. for 24 hours, and cool to room temperature. Filter, wash with water until neutral, and dry at 150°C for 24 hours. Under a nitrogen atmosphere, 80 mL of anhydrous toluene was added to the dry silica gel, and after stirring evenly, 2 mL of trimethoxymercaptopropylsilane and 0.5 mL of pyridine were added, and the mixture was refluxed and stirred at 110° C. for 24 hours. After the reaction system was cooled to room temperature, it was washed with toluene, methanol, water, tetrahydrofuran and methanol in sequence, and dried at 80° C. for 12 hours to obtain mercapto silica gel. Under a nitrogen atmosphere, add 30 mL of water and 1 g of maleimide biotin into a 50 mL three-necked round bottom flask, and stir until completely dissolved. 100 mg of catalyst [tris(2-car...
Embodiment 3
[0036] The difference from Example 1 is that 3-mercaptopropyltriethoxysilane is used instead of 3-mercaptopropyltrimethoxysilane, and the synthesis steps of Example 1 are followed to obtain a zwitterionic hydrophilic interaction chromatography stationary phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com