Method for preparing heteroatom co-doped porous carbon materials based on direct ionic liquid carbonization method
A technology of porous carbon materials and ionic liquids, applied in chemical instruments and methods, inorganic chemistry, carbon compounds, etc., can solve the problems of complex synthesis process, poor plasticity, and failure to meet the urgent requirements of environmental protection, and achieve simple synthesis process, Solve the effect of toxic and complex, excellent cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Mix 0.3g glucose, 50mg graphene oxide, 10mlBMIMHSO 4 The ionic liquid was sonicated for 3 hours, and then baked in an oven at 90°C for 12 hours. The obtained mixture was pre-sintered in a tube furnace, heated and heated under the protection of an inert gas at a heating rate of 5°C / min, a heat treatment temperature of 500°C, and kept for 1 hour to obtain a carbon precursor. Mix the carbon precursor and alkali metal potassium hydroxide at a mass ratio of 1:4, place the mixture in a tube furnace, and heat it up under the protection of an inert gas. The heating rate is 5°C / min, and the heat treatment temperature is 800°C , keep warm for 2h, and cool down to room temperature with the furnace. The activated product was washed with hydrochloric acid and deionized water, dried, and ground to obtain the target product.
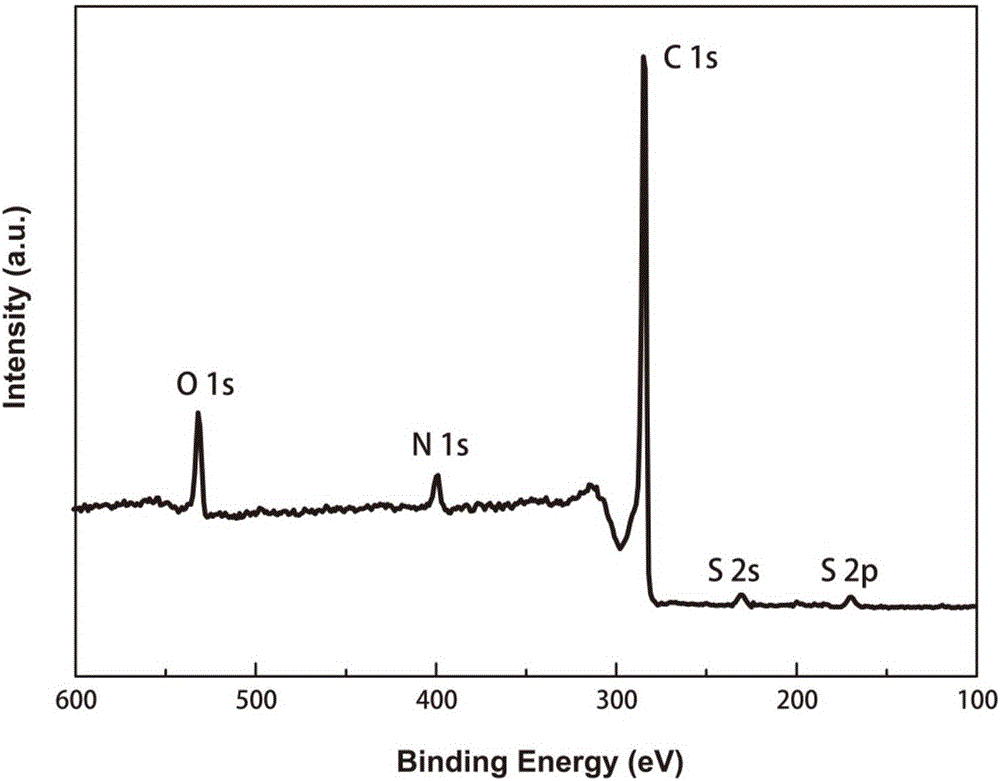
[0026] The structure was confirmed by X-ray photoelectron spectroscopy, such as figure 1 Shown is a nitrogen-sulfur co-doped porous carbon material.
[0027...
Embodiment 2
[0032] Mix 0.3g glucose, 50mg graphene oxide, 10mlBMIMHSO 4 The ionic liquid was sonicated for 3 hours, and then baked in an oven at 90°C for 12 hours. The obtained mixture was pre-sintered in a tube furnace, heated and heated under the protection of an inert gas at a heating rate of 5°C / min, a heat treatment temperature of 400°C, and kept for 2 hours to obtain a carbon precursor. Mix the carbon precursor and alkali metal potassium hydroxide at a mass ratio of 1:3, place the mixture in a tube furnace, and heat it up under the protection of an inert gas. The heating rate is 5°C / min, and the heat treatment temperature is 900°C , keep warm for 1h, and cool down to room temperature with the furnace. The activated product was washed with hydrochloric acid and deionized water, dried, and ground to obtain the target product.
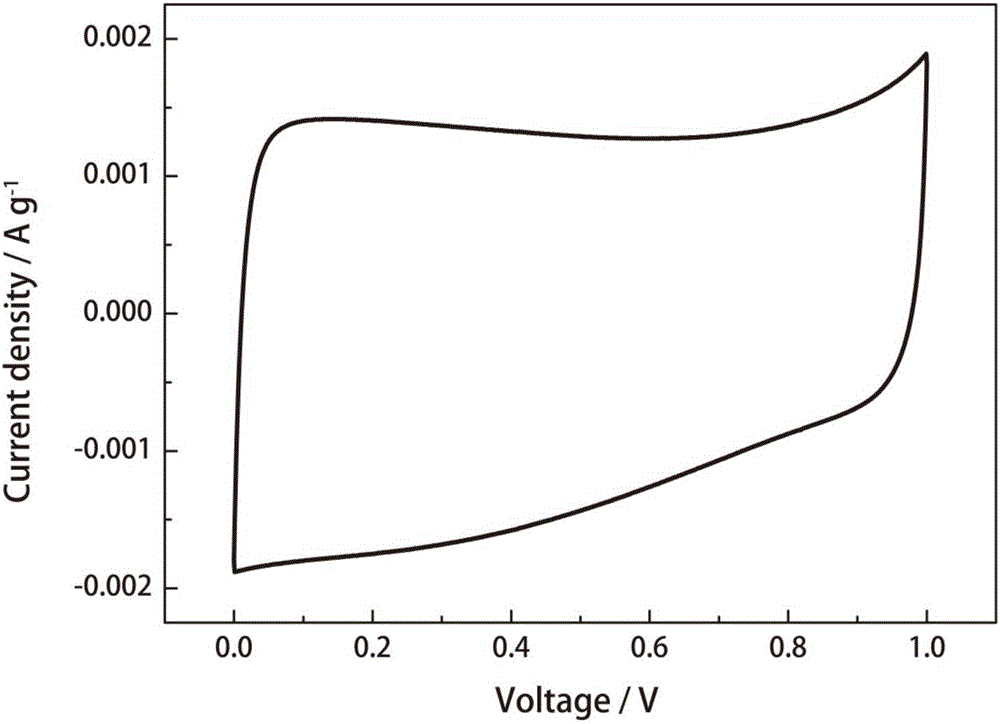
[0033] 10mV s -1 The results of the electrochemical performance tests are listed in Table 1.
Embodiment 3
[0035] Mix 0.3g glucose, 50mg graphene oxide, 10mlBMIMHSO 4 The ionic liquid was sonicated for 3 hours, and then baked in an oven at 90°C for 12 hours. The obtained mixture was pre-sintered in a tube furnace, heated and heated under the protection of an inert gas at a heating rate of 5°C / min, a heat treatment temperature of 300°C, and kept for 3 hours to obtain a carbon precursor. Mix the carbon precursor and alkali metal potassium hydroxide at a mass ratio of 1:1, place the mixture in a tube furnace, and heat it up under the protection of an inert gas. The heating rate is 5°C / min, and the heat treatment temperature is 700°C. , keep warm for 3h, and cool down to room temperature with the furnace. The activated product was washed with hydrochloric acid and deionized water, dried, and ground to obtain the target product.
[0036] 10mV s -1 The results of the electrochemical performance tests are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com