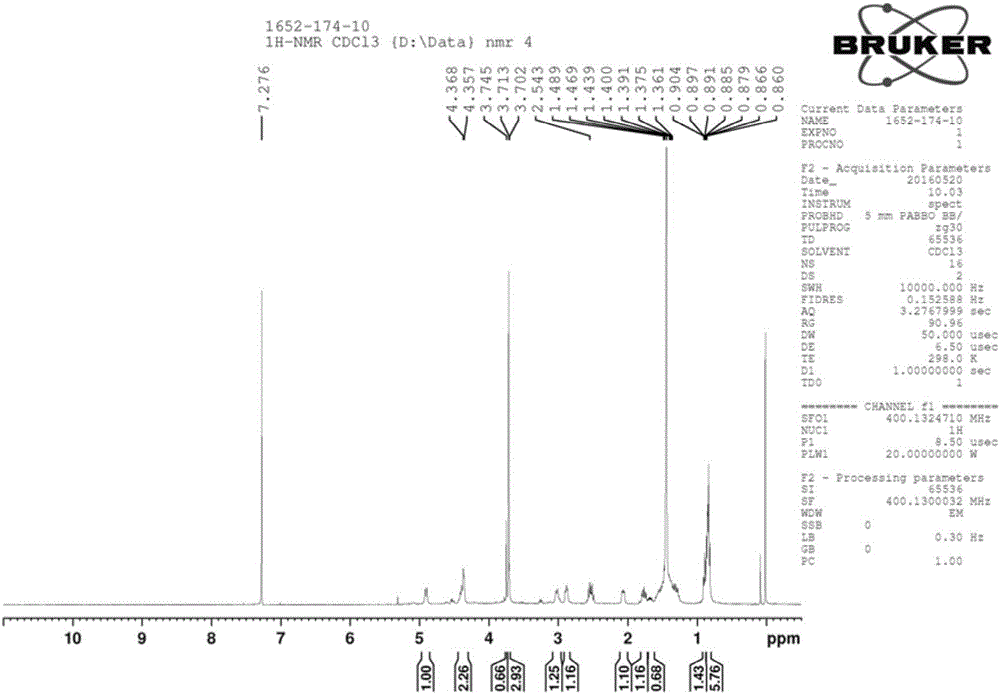
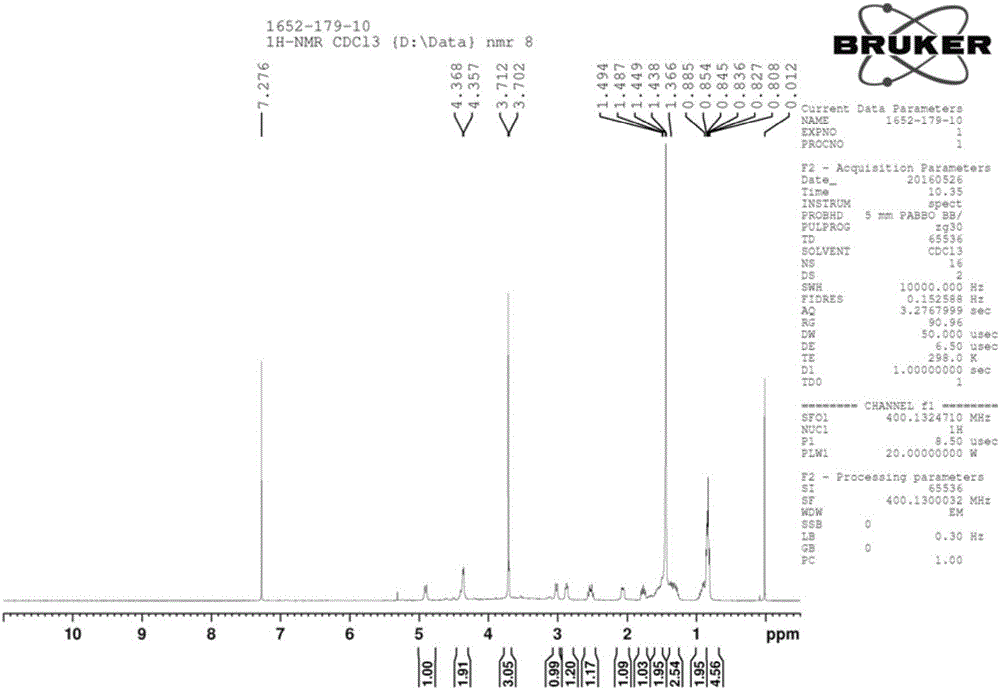
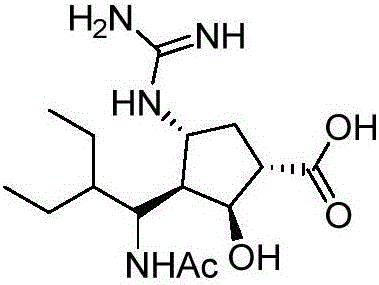
Method for preparing key intermediate of anti-influenza drug peramivir
An anti-influenza drug and intermediate technology, applied in the field of drug synthesis, can solve the problems of increasing the pressure of waste water discharge, scarcity and large amount of nickel ore, etc., and achieve the effects of reducing the pressure of the three wastes, high reaction conversion rate, and cheap use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Add compound A (1 mmol) and copper acetate monohydrate (0.5 mmol) into a 250 ml three-necked flask, dissolve with methanol, and cool to 0-5°C. Take another beaker, add sodium hydroxide (0.02mmol) and sodium borohydride (1.5mmol), dissolve in methanol and slowly drop into the three-necked flask, after the drop is complete, stir and react at room temperature for 12 hours. Samples were taken and the results analyzed by HPLC. In the post-treatment, ammonia water was added to quench the reaction, the solvent was spin-dried, and compound B was obtained by column chromatography.
Embodiment 2
[0034]
[0035] Add compound A (1 mmol) into a 250 ml three-necked flask, add nickel chloride hexahydrate (0.5 mmol), dissolve with methanol, and cool to 0-5°C. Take another beaker, add sodium hydroxide (0.02mmol) and sodium borohydride (1.5mmol), dissolve in methanol and slowly drop into the three-necked flask, after the drop is complete, stir and react at room temperature for 12 hours. Samples were taken and the results analyzed by HPLC. In the post-treatment, ammonia water was added to quench the reaction, the solvent was spin-dried, and compound B was obtained by column chromatography.
Embodiment 3
[0037]
[0038] Add compound A (1 mmol) into a 250 ml three-necked flask, add cobalt chloride hexahydrate (0.5 mmol), dissolve with methanol, and cool to 0-5°C. Take another beaker, add sodium hydroxide (0.02mmol) and sodium borohydride (1.5mmol), dissolve in methanol and slowly drop into the three-necked flask, after the drop is complete, stir and react at room temperature for 12 hours. Samples were taken and the results analyzed by HPLC. In the post-treatment, ammonia water was added to quench the reaction, the solvent was spin-dried, and compound B was obtained by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com