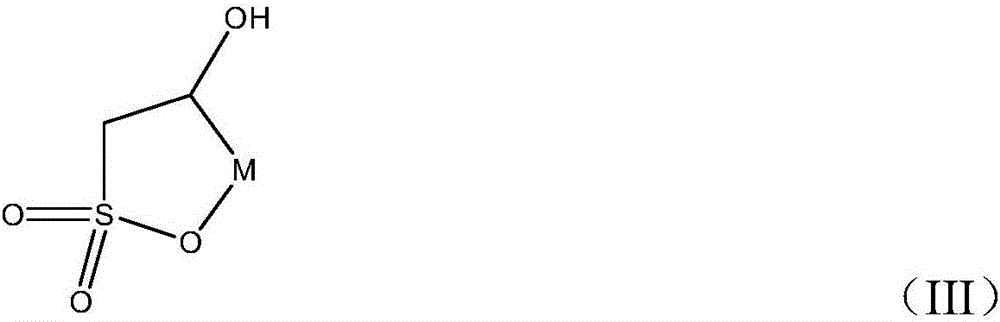Preparation method of fluorosultone
A fluorosulfonic acid, sultone technology, applied in the direction of organic chemistry, can solve the problems of difficult separation and purification, low synthesis yield, difficult reaction, etc., and achieve the effects of easy purification, short reaction steps and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0034] A preparation method of fluorosultone, is characterized in that, comprises the following steps:
[0035] (1), use saturated sodium sulfite aqueous solution and the chlorinated dihydroxyl compound generation substitution reaction with formula (I) structure, generate the dihydroxysulfonic acid sodium salt compound with formula (II) structure,
[0036]
[0037] M in formula (I) and formula (II) is C n h 2n+1 , wherein, n=1~4;
[0038] (2) The dihydroxysulfonic acid sodium salt compound obtained in step (1) is dehydrated with an excess dehydrating agent, the excess dehydrating agent is removed after the reaction, and the reaction product is washed and extracted to remove residual sodium dihydroxysulfonate Salt, then obtain the hydroxy-substituted sultone compound with formula (III) structure through drying and purification
[0039]
[0040] M of formula (III) is C n h 2n+1 , wherein, n=1~4;
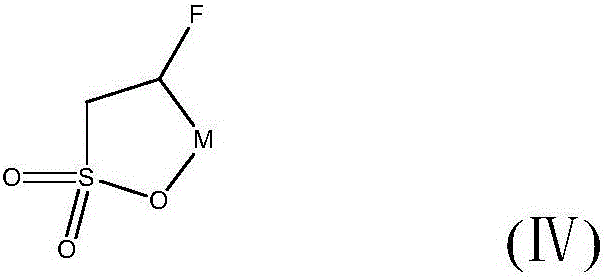
[0041] (3) The hydroxy-substituted sultone compound obtained in step (...
Embodiment 1
[0053] 2-fluoro-1,3-propane sultone
[0054] The reaction formula is as follows:
[0055]
[0056] Its preparation method comprises the following steps:
[0057](1), use saturated sodium sulfite aqueous solution and 3-chloro-1,2-propanediol to generate 2,3-dihydroxy-propanesulfonate sodium. Specifically, 33.2g (0.3mol) of 3-chloro-1,2-propanediol was slowly added dropwise to a reactor containing an aqueous solution of sodium sulfite (wherein the quality of sodium sulfite was 45.4g, and the molar weight was 0.36mol), and then Stir under reflux at 110° C. for 4 hours until the system becomes homogeneous, and a solution containing sodium 2,3-dihydroxy-propanesulfonate is obtained. The obtained solution of sodium 2,3-dihydroxy-propanesulfonate was concentrated to obtain a saturated solution, and then crystallized to obtain a solid product (hydrate) of sodium 2,3-dihydroxy-propanesulfonate.
[0058] (2) 2,3-dihydroxy-sodium propanesulfonate obtained in step (1) is subjected t...
Embodiment 2
[0060] Example 2: 3-fluoro-1,4-butane sultone
[0061] The reaction formula is as follows:
[0062]
[0063] Its preparation method comprises the following steps:
[0064] (1), use saturated sodium sulfite aqueous solution and 4-chloro-1,3-butanediol to generate 2,4-dihydroxy-sodium butanesulfonate. Specifically, 37.4g (0.3mol) of 4-chloro-1,3-butanediol was slowly added dropwise to a reactor filled with an aqueous solution of sodium sulfite (wherein the quality of sodium sulfite was 45.4g and the molar weight was 0.36mol), Then reflux and stir at 110° C. for 4 hours until the system became homogeneous, and a solution containing sodium 2,4-dihydroxy-butanesulfonate was obtained. The obtained solution of sodium 2,4-dihydroxy-butanesulfonate was concentrated to obtain a saturated solution, and then crystallized to obtain a solid product (hydrate) of sodium 2,4-dihydroxy-butanesulfonate.
[0065] (2) carrying out dehydration condensation reaction with 2,4-dihydroxy-sodium b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com