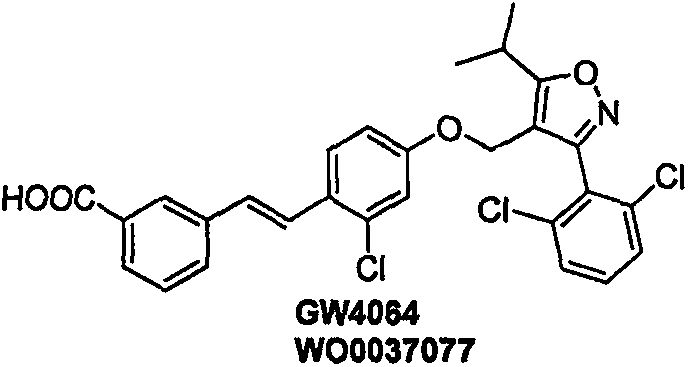
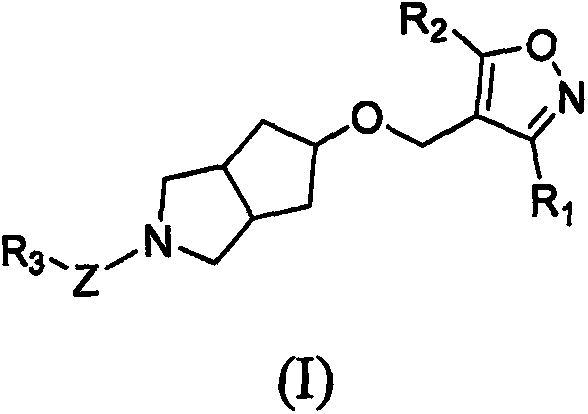
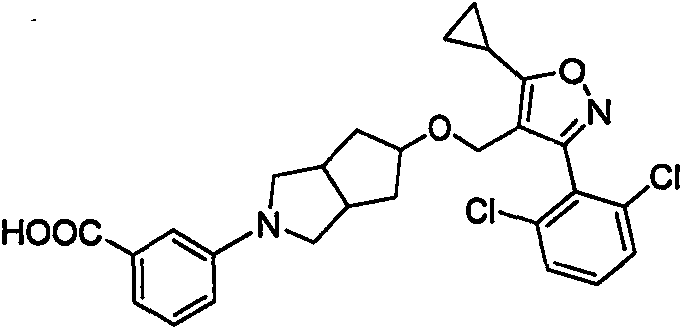
Spiro compound and medicinal use thereof
A technology for compounds and drugs, applied in the field of spiro compounds and their pharmaceutical uses, can solve the problems of short half-life, toxicity, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1. Preparation of 3-(5-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-position)methoxy)hexahydrocyclopenta[c]pyrrole -2(1H)-position)benzoic acid (01)
[0073]
[0074] Compound 01 was prepared according to the following procedure:
[0075]
[0076] Dissolve ketone (1.000g, 0.0044mol, 1eq) in 10mL of methanol, add sodium borohydride (0.503g, 0.0133mol, 3eq) in batches under ice bath, and react for 30min under ice bath after addition, TLC shows that the conversion of raw materials is complete, concentrate It was dried, quenched with water and dilute hydrochloric acid, extracted three times with ethyl acetate, the organic layer was combined and washed with water, washed with saturated sodium bicarbonate solution, washed with brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 0.990 g of off-white solid.
[0077] Intermediates 1-3:
[0078]
[0079] Add 1-2 (0.990g, 0.0044mol, 1.5eq) to 10mL of anhydrous tetrahydrofuran, ...
Embodiment 2
[0110] Compounds 2-7 were prepared according to the steps described in Example 1, and only the reaction raw materials were replaced accordingly to obtain the target compounds. Compound numbers, compound structures and HNMR / MS details are listed in Table 1:
[0111] Table 1 Compound number, compound structure and HNMR / MS results
[0112]
[0113]
Embodiment 3
[0114] Example 3 Farnesoid X receptor (FXR) activation test
[0115] Adopt FXR reporter gene method to test the activation activity of compound of the present invention, method is as follows:
[0116] I. Cell Culture
[0117] a. Trypsinization, seed cells at appropriate density in 10ml of complete matrix.
[0118] b. Incubate the cells for 24 hours at 37°C, 5% CO2.
[0119] II. Cell Seeding and Transfection
[0120] FuGENE HD was used as transfection reagent.
[0121] a. Prepare the transfection mixture according to the table below.
[0122] pBIND-FXR (ng / well) 25 pG5Luc(ng / well) 25 FuGENE HD(ul / well) 0.15 No FBS media(ul / well) 1.85 Total mix(ul / well) 2.5
[0123] b. Tap the tube vigorously to mix and incubate at room temperature for 15 minutes.
[0124] c. Trypsinization to measure cell density.
[0125] d. Dilute the cell solution to the required volume at a density of 600,000 cells / ml.
[0126] e. Add the required volume of transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com