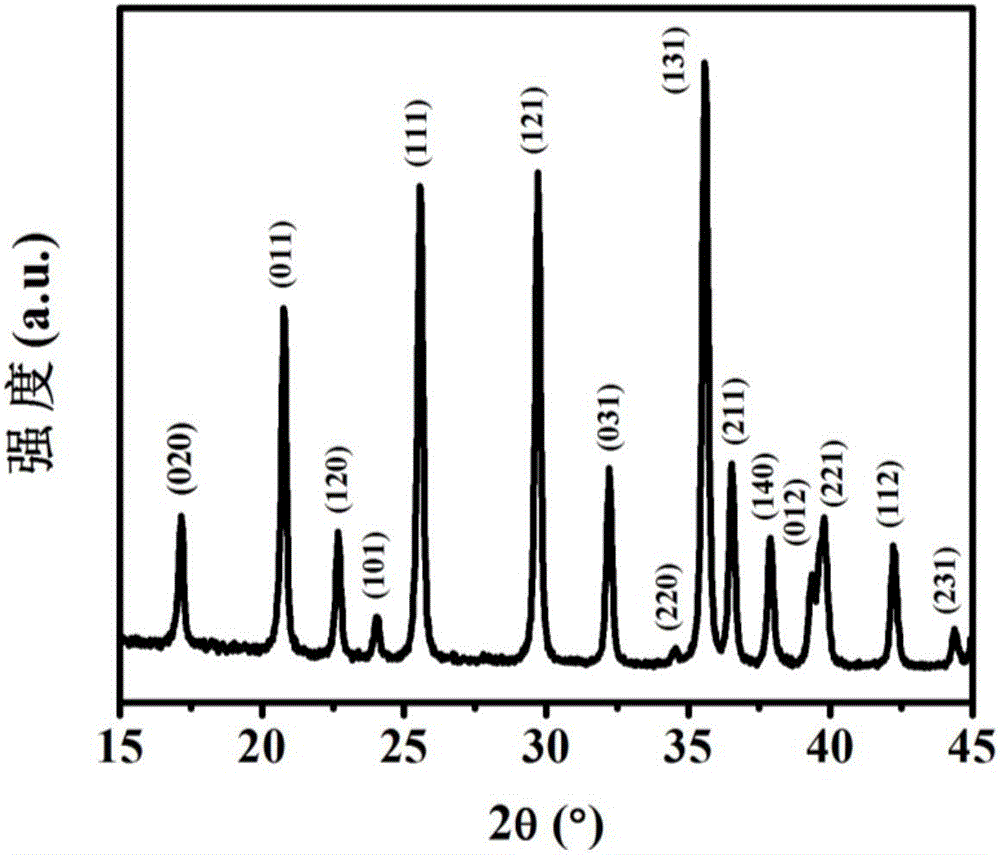
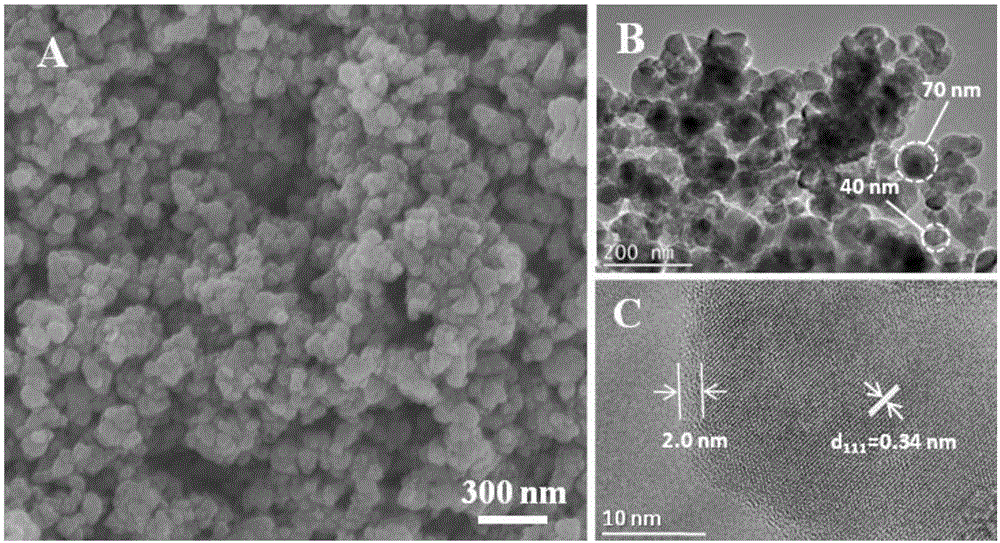
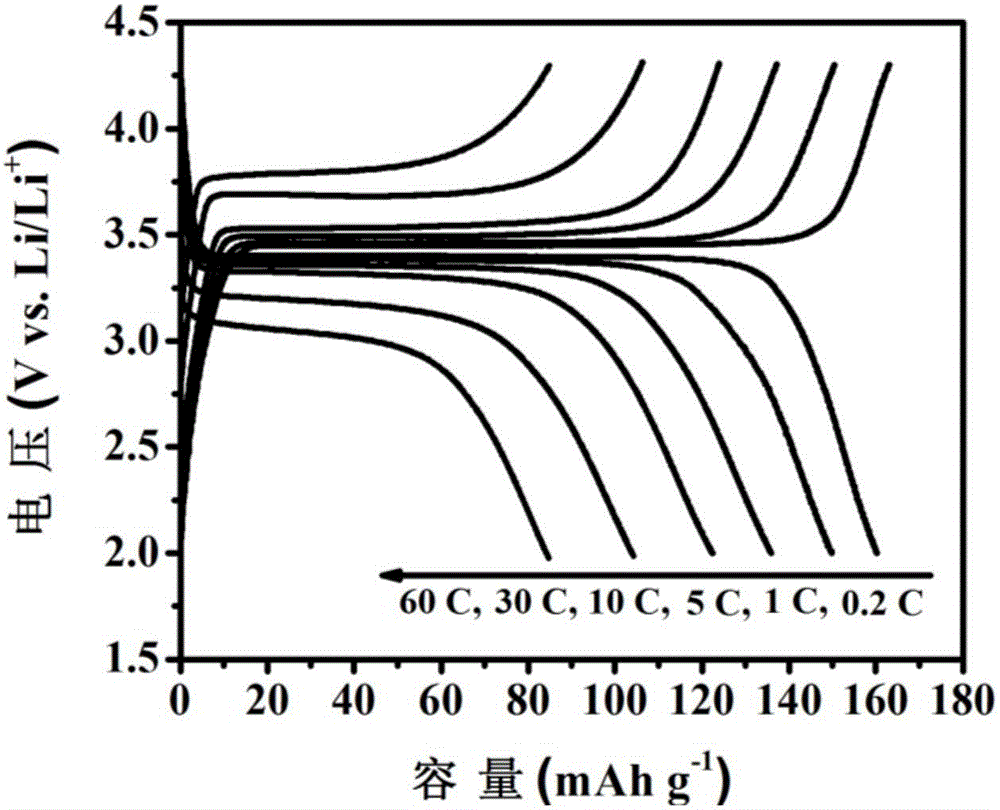
Ion exchange assisted preparation method of LiFePO4/C nano composite material
A nano-composite material and ion-exchange technology, which is applied in the field of ion-exchange-assisted preparation of LiFePO4/C nano-composite materials, can solve the problems of expensive reduction of lithiation agents, difficulty in popularization and promotion, etc., and achieve excellent electrochemical lithium storage performance, crystal grain Small and uniform, to achieve the effect of large-scale commercial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Weigh 18.5g FeCl 3 ·6H 2 O was dissolved in 300mL deionized water, stirred well, marked as solution A; weighed 7.86g NH 4 h 2 PO 4 Dissolve in 600mL deionized water, add 3mL aniline monomer, stir well, mark as solution B;
[0032] (2) With solution B under vigorous stirring (450rpm), slowly add solution A dropwise (2.5mL min -1 ), and aged for 5 h with stirring at room temperature. After the reaction, the precipitated product was suction filtered, washed with deionized water, and dried (60°C, 24h) to obtain amorphous FePO 4 ·xH 2 O / PANI powder precursor;
[0033] (3) Weigh 2.325g of the powder precursor obtained in step (2) and uniformly disperse it into 60mL of lithium acetate ethanol solution with a concentration of 0.6M, and lithiate at 60°C for 2h with constant temperature stirring (150rpm). After the system was cooled, the product was subjected to suction filtration, washed with ethanol, and dried at 60°C for 2 hours to obtain Li-Fe(III)PO 4 / PANI lith...
Embodiment 2
[0036] (1) Weigh 2.703g FeCl 3 ·6H 2 O was dissolved in 50mL deionized water, stirred well, marked as solution A; weighed 1.15g NH 4 h 2 PO 4 Dissolve in 100mL deionized water, stir well, and mark it as solution B;
[0037] (2) With solution B under strong stirring (600rpm), slowly add solution A dropwise (1.5mL min -1 ), and aging with stirring at room temperature for 0.5h. After the reaction, the precipitated product was suction filtered, washed with deionized water, and dried (50°C, 12h) to obtain amorphous FePO 4 ·xH 2 O powder precursor;
[0038] (3) Weigh 0.3 g of the powder precursor obtained in step (2) and uniformly disperse it into 10 mL of lithium acetate ethanol solution with a concentration of 0.3 M, and lithiate at 65° C. with constant temperature stirring (180 rpm) for 2 h. After the system was cooled, the precipitated product was suction filtered, washed with ethanol, and dried at 60°C for 1.5h to obtain Li-Fe(III)PO 4 Lithiated intermediates;
[0039...
Embodiment 3
[0041] (1) Weigh 4.04g Fe(NO 3 ) 3 9H 2 O was dissolved in 50mL deionized water, stirred well, marked as solution A; weighed 1.15g NH 4 h 2 PO 4 Dissolve in 100mL deionized water, add 0.5mL aniline monomer, stir well, and mark it as solution B;
[0042] (2) With solution B under strong stirring (600rpm), slowly add solution A dropwise (2.0mL min -1 ), and aged with stirring at room temperature for 8h. After the reaction, the precipitated product was suction filtered, washed with deionized water, and dried (60°C, 10h) to obtain amorphous FePO 4 ·xH 2 O / PANI powder precursor;
[0043] (3) Weigh 0.2g of the powder precursor obtained in step (2) and uniformly disperse it into 10mL of lithium acetate ethanol solution with a concentration of 0.2M, and lithiate at 60°C for 2.5h with constant temperature stirring (150rpm). After the system was cooled, the product was subjected to suction filtration, washed with ethanol, and dried at 60°C for 2 hours to obtain Li-Fe(III)PO 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com