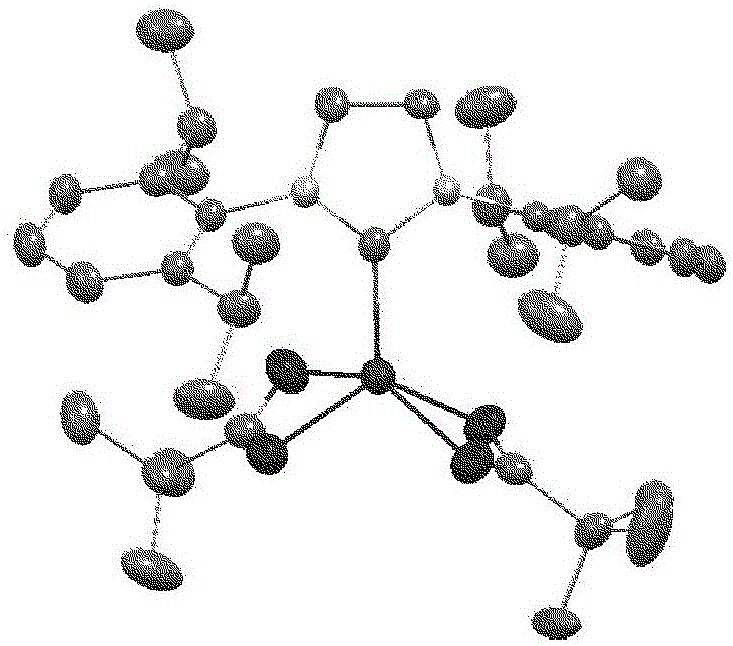
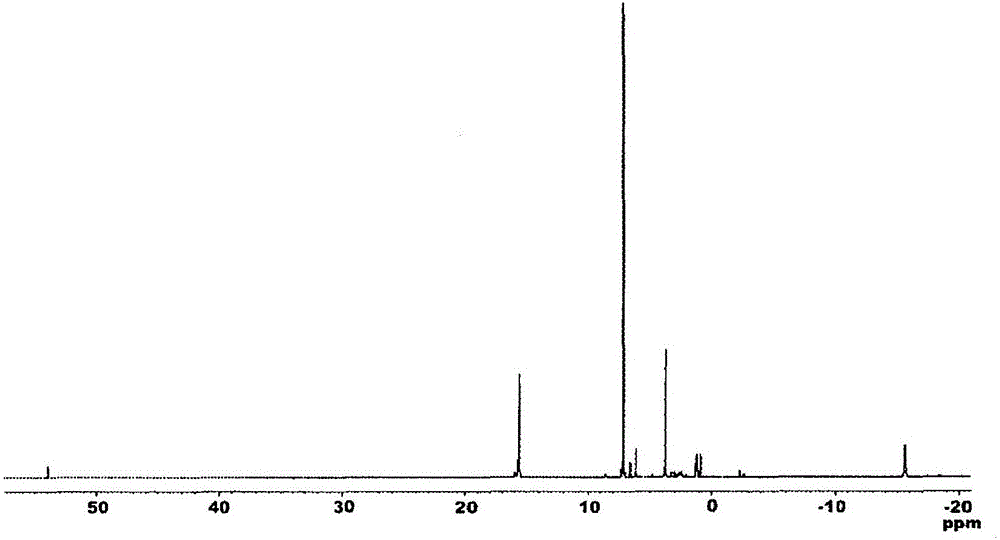
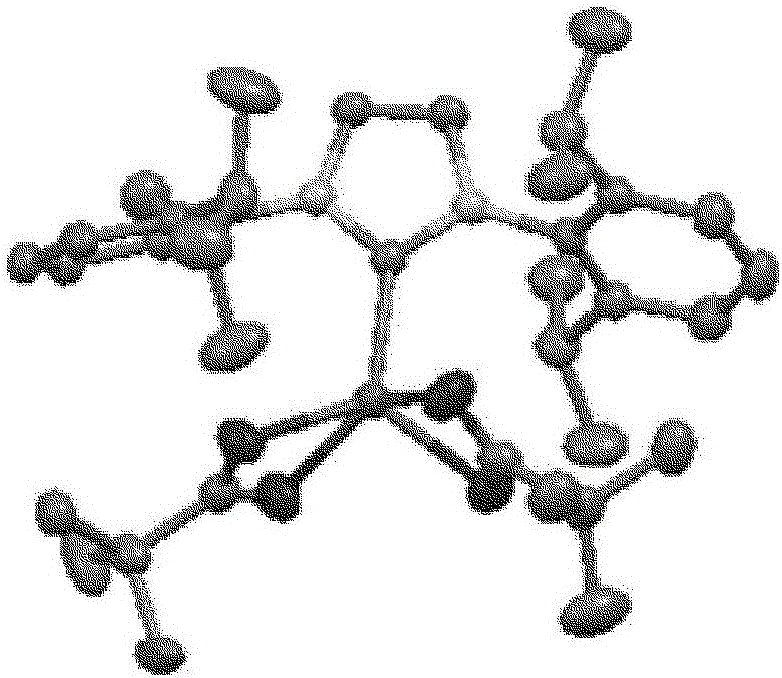
Hydrosilylation reaction catalyst
一种氢化硅烷化、催化剂的技术,应用在催化反应、物理/化学过程催化剂、有机化合物/氢化物/配位配合物催化剂等方向,能够解决配位化合物稳定性低、烯烃反应性低、没有显示催化剂活性等问题,达到有用性高、反应活性高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0237] The following synthesis examples and examples are given to describe the present invention more specifically, but the present invention is not limited by the following examples.
[0238] Synthesis of complexes was performed using Schneenck technique or a glove box under a nitrogen or argon atmosphere. All solvents used in the preparation of metal compounds were deoxidized and dehydrated by known methods.
[0239] The obtained metal compound was stored at 25°C under a nitrogen atmosphere and used in the reaction.
[0240] The hydrosilylation reaction and solvent purification of olefins were all carried out under an inert gas atmosphere, and solvents used in various reactions were all purified, dried, and deoxidized by known methods in advance.
[0241] 1 H. 13 C-NMR measurement was performed using JNM-ECA600 and JNM-LA400 manufactured by JEOL Ltd., IR measurement was performed using FT / IR-550 manufactured by JASCO Corporation, and elemental analysis was performed using ...
Synthetic example 1
[0244] [Synthesis example 1] Synthesis of iron pivalate
[0245] Taking the literature J.Cluster.Sci., 2005, 16, 331. as a reference, it was synthesized by the following method.
[0246]0.86 g (15.4 mmol) of reduced iron and 3.50 g (34.3 mmol) of pivalic acid were added to a 50 mL two-necked eggplant-shaped flask equipped with a reflux tube, and stirred at 160° C. for 12 hours. At this time, the reaction solution changed from colorless and transparent to green. Furthermore, 2.50 g (24.5 mmol) of pivalic acid was added, and it stirred at 160 degreeC for 19 hours. Then, the reaction solution was filtered, combined with the recovered supernatant, and dried under reduced pressure at 80°C. The obtained solid was washed with hexane to obtain a green solid (2.66 g, yield 67%).
[0247] FT-IR (KBr) ν: 2963, 2930, 2868, 1583, 1523, 1485, 1457, 1427, 1379, 1362, 1229, 1031, 938, 900, 790, 608, 576, 457cm- 1
Synthetic example 2
[0248] [Synthesis example 2] used [Fe( base) (μ- base)] 2 Preparation of iron precursors with iron-oxygen bonds
[0249] With reference to the document Organometallics, 1993, 12, 2414, it was synthesized by the following method.
[0250] 1.08 g (44.3 mmol) of magnesium bars and 35 mL of THF were placed in a 50 mL two-necked eggplant-shaped flask, and 8.49 g (42.6 mmol) of brominated mesitylene was slowly added dropwise thereto. After the dropwise addition, after confirming the generation of heat generation, the mixture was stirred at 60° C. for 3 hours. The resulting solution was filtered through a glass filter to prepare the bromo A solution of magnesium-based Grignard reagent in THF.
[0251] Add FeCl to a 100 mL Schnencke tube 2 2.63g (20.7mmol), THF30mL, 1,4-dioxane 10mL, cooled to -78°C. Slowly add the above-prepared bromo A THF solution of magnesium-based Grignard reagent was stirred at 25°C for 2 hours. At this time, the reaction solution changed from a bro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com