Preparation method of solid acid catalyst for preparing 5-hydroxymethylfurfural from biomass
A solid acid catalyst, a technology of hydroxymethyl furfural, which is applied in the fields of catalytic chemistry and biomass resource utilization, can solve the problems of poor catalyst stability, decreased reaction activity, and is not suitable for large-scale production, and achieves stable structure and good water resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-18
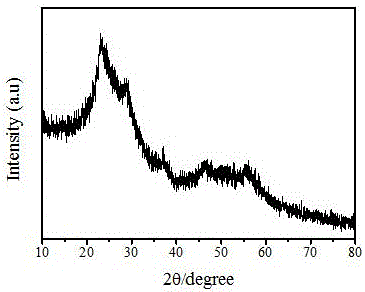
[0030] Weigh a certain amount of carbon source and 2 grams of niobium source into a beaker, add it to 20mL acidic solution, stir continuously for 2 hours, transfer the obtained solution to a stainless steel autoclave lined with polytetrafluoroethylene, and crystallize at a certain temperature After some time a dark brown solid was obtained. After washing with deionized water, drying, and then placing in a tube furnace, carbonizing for a certain period of time in a nitrogen atmosphere and at a certain temperature, the required niobium-carbon solid acid catalyst (carbon source, niobium source, mass ratio , synthesis solvent, and carbonization temperature are shown in Table 1, and the physicochemical parameter characterization results of each solid acid catalyst are shown in Table 2).
[0031] Add 0.2 g of catalyst, 0.2 g of cellulose, 6 mL of tetrahydrofuran, and 2 mL of saturated aqueous sodium chloride into a high-pressure magnetic stirring batch reactor, fill with 0.5 MPa nit...
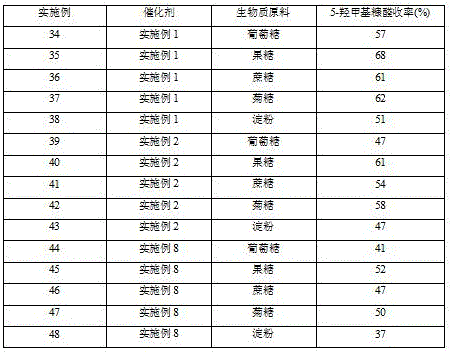
Embodiment 34-48
[0033] Add 0.2 g of the catalyst in the above example, 0.2 g of biomass raw material, 6 mL of tetrahydrofuran, 2 mL of saturated aqueous sodium chloride solution into a high-pressure magnetic stirring batch reaction kettle, fill with 0.5 MPa nitrogen, heat to 180 ° C, and react at constant temperature for 8 hours , the reaction system was cooled to room temperature (25°C), and the catalyst was separated by centrifugation. The reaction solution was analyzed by high performance liquid chromatography, and the yield of 5-hydroxymethylfurfural was calculated, as shown in Table 3.
Embodiment 49
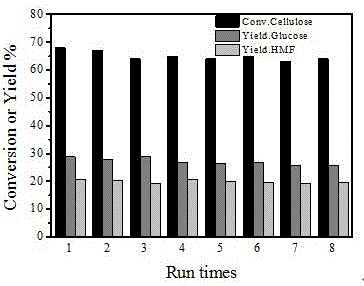
[0035] Add 0.2 g of the catalyst of Example 1, 0.2 g of cellulose, 6 ml of tetrahydrofuran, and 2 ml of saturated aqueous sodium chloride into a high-pressure magnetic stirring batch reactor, fill with 0.5 MPa nitrogen, heat to 180 ° C, and react at a constant temperature for 8 hours. The reaction system was cooled to room temperature (25°C), and the catalyst was separated by centrifugation. The reaction solution was analyzed by high performance liquid chromatography, and the yield of 5-hydroxymethylfurfural was calculated. The catalyst was put into the next reaction after being washed with deionized water, and a total of 8 reactions were used in a cycle. The results of each reaction are shown in the attached figure 2 , it can be seen that the niobium-carbon solid acid catalyst exhibits good cycle stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com